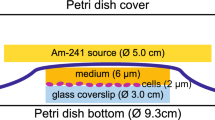
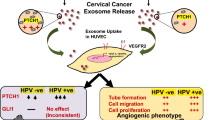
Abstract
This study was conducted to investigate the inhibitory effects of light-emitting diodes (LEDs) on exosome biogenesis and angiogenesis capacity in Ishikawa endometrial cancer cells. To this end, cells were exposed to different energy densities (fluences) of 4, 8, 16, 32, and 64 J/cm2 for 5 days (once every 24 h), and cell viability was determined using an MTT assay. Based on data from the MTT panel, cells were exposed to 4 and 16 J/cm2 for subsequent analyses. Exosome biogenesis was also monitored via monitoring the expression of CD63, ALIX, and Rab27a and b. The size and morphology of exosomes in the supernatant were measured using scanning electron microscopy (SEM), and dynamic light scattering (DLS). Using Transwell insert, the migration capacity of these cells was studied. The angiogenic effects of irradiated Ishikawa cell secretome at different fluences were monitored on human endothelial cells using in vitro tubulogenesis. Results indicated LED can reduce the viability of Ishikawa cells in a dose-dependent manner. According to our data, 4 and 64 J/cm2 groups exhibited minimum and maximum cytotoxic effects compared to the control cells. Data revealed a close proportional relationship between the power of laser and exosome average size compared to the non-treated control cells (p < 0.05). Real-time PCR analysis showed the suppression of Rab27b and up-regulation of Rab27a in irradiated cells exposed to 4 and 16 J/cm2 (p < 0.05). These effects were evident in the 16 J/cm2 group. Likewise, LED can inhibit the migration of Ishikawa cells in a dose-dependent manner (p < 0.05). Tubulogenesis activity of endothelial cells was suppressed after incubation with the secretome of irradiated Ishikawa cells (p < 0.05). These data showed tumoricidal properties of LED irradiation on human adenocarcinoma Ishikawa cells via the inhibition of exosome biogenesis and suppression of angiogenesis capacity.





Similar content being viewed by others
References
Davey MG, Miller N, McInerney NM (2021) A review of epidemiology and cancer biology of malignant melanoma. Cureus 18;13(5):e15087
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Passaro A et al (2022) Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J Clin Oncol 40(6):598–610
Asadi S et al (2021) Laser-induced optothermal response of gold nanoparticles: from a physical viewpoint to cancer treatment application. J Biophotonics 14(2):e202000161
Arjmand B et al (2021) Low-level laser therapy: potential and complications. J Lasers Med Sci 12:e42. https://doi.org/10.34172/jlms.2021.42
Khalkhal E et al (2020) Evaluation of laser effects on the human body after laser therapy. J Lasers Med Sci 11(1):91
Rohringer S et al (2017) The impact of wavelengths of LED light-therapy on endothelial cells. Sci Rep 7(1):1–11
Mohammadizadeh M, Eliadarani FK, Badiei Z (2012) Is the light-emitting diode a better light source than fluorescent tube for phototherapy of neonatal jaundice in preterm infants? Adv Biomed Res 1:51. https://doi.org/10.4103/2277-9175.100158
Asnaashari M et al (2016) A comparison of the antibacterial activity of the two methods of photodynamic therapy (using diode laser 810 nm and LED lamp 630 nm) against Enterococcus faecalis in extracted human anterior teeth. Photodiagn Photodyn Ther 13:233–237
Srivastava A et al (2016) Exploitation of exosomes as nanocarriers for gene-, chemo-, and immune-therapy of cancer. J Biomed Nanotechnol 12(6):1159–1173
Munagala R et al (2016) Bovine milk-derived exosomes for drug delivery. Cancer Lett 371(1):48–61
Johnsen KB et al (1846) (2014) A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta Rev Cancer 1:75–87
Heidarzadeh M et al (2021) Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci 11(1):142
Rezabakhsh A, Sokullu E, Rahbarghazi R (2021) Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther 12(1):521
Vlassov AV et al (1820) (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta Gen Subj 7:940–948
Bobrie A et al (2011) Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12(12):1659–1668
Ostrowski M et al (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12(1):19–30
Fader CM et al (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta Mol Cell Res 1793(12):1901–1916
Schwartz SL et al (2007) Rab GTPases at a glance. J Cell Sci 120(22):3905–3910
Barr F, Lambright DG (2010) Rab gefs and gaps. Curr Opin Cell Biol 22(4):461–470
Fukuda M (2011) TBC proteins: GAPs for mammalian small GTPase Rab? Biosci Rep 31(3):159–168
Valcz G et al (2016) Exosomes in colorectal carcinoma formation: ALIX under the magnifying glass. Mod Pathol 29(8):928–938
Men Y et al (2019) Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun 10(1):1–18
Pournaghi M et al (2021) Effect of melatonin on exosomal dynamics in bovine cumulus cells. Process Biochem 106:78–87
Rezabakhsh A, Sokullu E, Rahbarghazi R (2021) Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther 12(1):1–8
Bagheri HS et al (2018) Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med Sci 33(5):1131–1145
Bagheri HS et al (2020) Low-level laser irradiation modulated viability of normal and tumor human lymphocytes in vitro. J Lasers Med Sci 11(2):174
Khorsandi K, Kianmehr Z, Hosseinzadeh R (2020) Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int 20(1):1–14
Fukuda M (2013) Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 14(9):949–963
Wei H et al (2021) Regulation of exosome production and cargo sorting. Int J Biol Sci 17(1):163
Gruenberg J, Van der Goot FG (2006) Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol 7(7):495–504
Gao G et al (2019) From perinuclear to intranuclear localization: a cell-penetrating peptide modification strategy to modulate cancer cell migration under mild laser irradiation and improve photothermal therapeutic performance. Biomaterials 223:119443
Bao Y-W et al (2018) Platinum-doped carbon nanoparticles inhibit cancer cell migration under mild laser irradiation: multi-organelle-targeted photothermal therapy. Biomaterials 183:30–42
Acknowledgements
The authors would like to thank the special assistance from the Biophotonics Research Center of Tabriz Islamic Azad University for the design and development of the LED chamber. Also, we would like to show our sincere gratitude to the personnel of Stem Cell Research Center, Tabriz University of Medical Sciences, for providing a unique environment for performing the experiments.
Funding
This study was supported by a grant from Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Sima Mojtahedin, Fatemeh Sokouti Nasimi, Habib Tajalli, Soheila Ebrahimi, and Behrad Alimohammadzadeh performed analysis and prepared manuscript. Reza Rahbarghazi and Mahdi Mahdipour supervised the study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All phases of this study were approved by the Local Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1399.444).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mojtahedin, S., Nasimi, F.S., Tajalli, H. et al. Light-emitting diode photomodulation of uterine adenocarcinoma cells inhibited angiogenesis capacity via the regulation of exosome biogenesis. Lasers Med Sci 37, 3193–3201 (2022). https://doi.org/10.1007/s10103-022-03597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-022-03597-x