Abstract
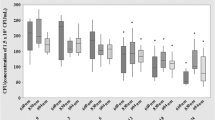
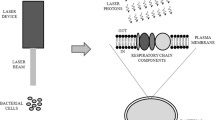
We investigated the influence of femtosecond laser irradiation on the growth of the two most common infectious bacterial pathogens in wounds; Staphylococcus aureus and Pseudomonas aeruginosa as an attempt to validate optimum parameters for a laser-based bactericidal modality to be used clinically. Bacterial cultures were exposed to femtosecond laser irradiation at different wavelengths, exposure times, and laser powers. The source of femtosecond laser was INSPIRE HF100 laser system, Spectra-Physics, which is pumped by a mode-locked femtosecond Ti: sapphire laser MAI TAI HP, Spectra-Physics. After irradiation, bacterial cells’ survival was monitored by observing the clear zones of inhibition in cultured agar plates. Results for all strains indicated that the exposure to femtosecond laser irradiation with a wavelength ranging from ultraviolet (λ > 350 nm) to blue laser light (λ < 480 nm), for a period above 20 min and with a power density of ≈ 0.063 W/cm2, was enough to inhibit both bacterial pathogens with the results maintained for 1 week following irradiation.





Similar content being viewed by others
References
Heldin C-H, Westermark B (1988) Role of platelet-derived growth factor in vivo. Springer, The molecular and cellular biology of wound repair
Stacey M (2016) Why don’t wounds heal?. Wounds Int. 7(1):16–21
Bowler PG, Duerden BI, Armstrong DG (2001) Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 14(2):244–269
Fagbomedo J, Femi TO (2017) Incidence of wound infections and the prevalence of multi drug resistant Staphylococcus aureus in a Nigerian Hospital. Academia J Sci Res 5:316–322
Isibor JO, Oseni A, Eyaufe A, Turay A (2008) Incidence of aerobic bacteria and Candida albicans in postoperative wound infections. Afr J Microbiol Res 2:288–291
Smith RS, Iglewski BH (2003) P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 6(1):56–60
Wang J, Wu X, Wang C, Rong Z, Ding H, Li H et al (2016) Facile synthesis of Aucoated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl Mater Interfaces 8(31):19958–19967
Anguzu J, Olila D (2007) Drug sensitivity patterns of bacterial isolates from septic postoperative wounds in a regional referral hospital in Uganda. Afr Health Sci 7
Gelaw A, Gebre-Selassie S, Tiruneh M, Mathios E, Yifru S (2014) Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at the University of Gondar Hospital, Northwest Ethiopia. J Environ Occup Sci 3:103–108
Kramer A, Schwebke I, Kampf G (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6:130
Luo J, Deng W, Yang F, Wu Z, Huang M, Gu M (2018) Gold nanoparticles decorated graphene oxide/nanocellulose paper for NIR laser-induced photothermal ablation of pathogenic bacteria. Carbohydr Polym 198:206–214
Gravitz L (2012) Turning a new phage. Nat Med 18:1318–1320
Pelfrene E et al (2016) Bacteriophage therapy: a regulatory perspective. J ntimicrob Chemother 71:2071–2074
Mahlapuu M et al (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Cotter PD et al (2013) Bacteriocins – a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105
Yang SC et al (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:241
Isolauri E et al (2002) Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 50:III54–III59
Aroniadis OC, Brandt LJ (2014) Intestinal microbiota and the efficacy of fecal microbiota transplantation in gastrointestinal disease. Gastroenterol Hepatol (N Y) 10:230–237
Kadouri DE et al (2013) Predatory bacteria: a potential ally against multidrug-resistant Gram-negative pathogens. PLoS One 8:e63397
Saylor C et al (2009) Monoclonal antibody-based therapies for microbial diseases. Vaccine 27:G38–G46
Bebbington C, Yarranton G (2008) Antibodies for the treatment of bacterial infections: current experience and future prospects. Curr Opin Biotechnol 19:613–619
Scorciapino MA, Rinaldi AC (2012) Antimicrobial peptidomimetics: reinterpreting nature to deliver innovative therapeutics. Front Immunol 3:171
Ayhan DH et al (2016) Sequence-specific targeting of bacterial resistance genes increases antibiotic efficacy. PLoS Biol 14:e1002552
Meng J et al (2009) Novel anion liposome-encapsulated antisense oligonucleotide restores susceptibility of methicillin-resistant Staphylococcus aureus and rescues mice from lethal sepsis by targeting mecA. Antimicrob Agents Chemother 53:2871–2878
Meng J et al (2015) Reversion of antibiotic resistance by inhibiting mecA in clinical methicillin-resistant staphylococci by antisense phosphorothioate oligonucleotide. J Antibiot (Tokyo) 68:158–164
Chandradhish G et al (2018) Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol 27(4)
Lu TK, Collins JJ (2007) Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A 104:11197–11202
Libis VK et al (2014) Silencing of antibiotic resistance in E. coli with engineered phage bearing small regulatory RNAs. ACS Synth Biol 3:1003–1006
Nelson DC et al (2012) Endolysins as antimicrobials. Adv Virus Res 83:299–365
Schmelcher M et al (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171
Borysowski J et al (2006) Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood) 231:366–377
Rodriguez-Rubio L et al (2013) Bacteriophage virion-associated peptidoglycan hydrolases: potential new enzybiotics. Crit Rev Microbiol 39:427–434
De La Fuente-Nunez C, Lu TK (2017) CRISPR-Cas9 technology: applications in genome engineering, development of sequence-specific antimicrobials, and future prospects. Integr Biol (Camb) 9:109–122
Bikard D, Barrangou R (2017) Using CRISPR-Cas systems as antimicrobials. Curr Opin Microbiol 37:155–160
Kokai-Kun JF et al (2017) The oral beta-lactamase SYN-004 (Ribaxamase) degrades ceftriaxone excreted into the intestine in phase 2a clinical studies. Antimicrob Agents Chemother 61:e02197–e02116
Pereira PR, DE Paula JB, Cielinski J, Pilonetto M, VON Bahten LC (2014) Effects of low intensity laser in in vitro bacterial culture and in vivo infected wounds. Rev Col Bras Cir 41(1):49–55
Amin RM, Bhayana B, Hamblin MR, Dai T (2016) Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: in vitro and in vivo studies. Lasers Surg Med 48:562–568
Samaneh R, Ali Y, Mostafa J, Mahmud NA, Zohre R (2015) Laser therapy for wound healing: a review of current techniques and mechanisms of action. Biosci, Biotech Res Asia 12:217–223
Hamblin MR, Abrahamse H (2019) Can light-based approaches overcome antimicrobial resistance? Drug Dev Res 80:48–67
Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ (2005) Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother 49:2822–2827
Dia T, Gupta A, Murrayd CK, Vrahase MS, Tegosa GP, Hamblin MR (2012) Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat 15:223–236
Ferro S, Ricchelli F, Monti D, Mancini G, Jori G (2007) Efficient photoinactivation of methicillinresistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int J Biochem Cell Biol 39:1026–1034
Maclean M, Macgregor SJ, Anderson JG, Woolsey G (2009) Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light emitting diode array. Appl Environ Microbiol 75:1932–1937
Maclean M, Macgregor SJ, Anderson JG, Woolsey GA (2008) The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B Biol 92:180–184
Dai T, Garcia B, Murray CK, Vrahas MS, Hamblin MR (2012) UVC light prophylaxis for cutaneous wound infections in mice. Antimicrob Agents Chemother 56:3841–3848
Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC, Dai T (2017) Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resist Updat 33-35:1–22
Ziegelberger G (2013) ICNIRP Guidelines on limits of exposure to laser radiation of wavelengths between 180 nm and 1,000 μm. Health Phys 105(3):271–295
Morita S, Tagai C, Shiraishi T, Miyaji K, Iwamuro S (2013) Differential mode of antimicrobial actions of arginine-rich and lysine-rich histones against gram-positive Staphylococcus aureus. Peptides 48:75–82
Nitzan Y, Salmon-Divon M, Shporen E, Malik Z (2004) ALA induced photodynamic effects on gram positive and negative bacteria. Photochem Photobiol Sci 3:430–435
Ash C, Dubec M, Donne K, Bashford T (2017) Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci 32(8):1909–1918. https://doi.org/10.1007/s10103-017-2317-4
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. In Seminars in cutaneous medicine and surgery (Vol. 32, No. 1, p. 41). NIH Public Access
Jelínková H (2013) Lasers for medical applications: diagnostics, therapy and surgery. Elsevier
Krespi YP, Stoodley P, Hall-Stoodley L (2008) Laser disruption of biofilm. Laryngoscope 118(7):1168–1173
Taylor ZD, Navarro A, Kealey CP, Beenhouwer D, Haake DA, Grundfest WS, Gupta V (2010) Bacterial biofilm disruption using laser generated shockwaves. In 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology (pp. 1028-1032). IEEE
Polikov, V., Block, M., Zhang, C., Reichert, W. M., & Hong, J. S. 2011. In vitro models for neuroelectrodes: a paradigm for studying tissue–materials interactions in the brain
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Research does not contain any studies involving human participants and/or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, E., El-Gendy, A.O., Moniem Radi, N.A. et al. The bactericidal efficacy of femtosecond laser-based therapy on the most common infectious bacterial pathogens in chronic wounds: an in vitro study. Lasers Med Sci 36, 641–647 (2021). https://doi.org/10.1007/s10103-020-03104-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03104-0