Abstract
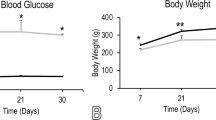
Pathophysiologic conditions associated with diabetes mellitus affect mesenchymal stem cells (MSCs), and this phenomenon may lead to some diabetic secondary complications. The present study was conducted to evaluate the impact of photobiomodulation (PBM) on rat diabetic MSC (DMSC) behavior in vitro. For the purpose of PBM, we used helium-neon laser with a wavelength of 632.8 nm at three different energy densities (0.5, 1, 2 J/cm2) and radiation periodicity of once, twice, and thrice. The survival, proliferation, and apoptosis in the normal MSCs (NMSCs), DMSCs, and diabetic MSCs, which were laser irradiated (DMSCs+L), were assessed using MTT assay, Ki67 immunofluorescence staining, and TUNEL assay, respectively. Our results demonstrated that DMSCs have significantly lower survival (P < 0.05) and proliferation rates (P < 0.001), and dramatically higher population doubling time (PDT, P < 0.001) and apoptosis rates (P < 0.001) as compared to NMSCs. Moreover, PBM with energy density of 1 J/cm2 and the periodicity of 1 or 2 times could improve diabetic MSC capabilities in the term of survival, proliferation, and apoptosis. Considering these findings, it is suggested that PBM could improve the ability of diabetic MSCs in vitro prior to transplantation or may rise their capabilities in their native niche in vivo.





Similar content being viewed by others
References
Guariguata L et al (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149
Ogurtsova K et al (2017) IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50
Mathis D, Vence L, Benoist C (2001) Beta-cell death during progression to diabetes. Nature 414(6865):792–798
Davey GC et al (2014) Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol. https://doi.org/10.3389/fendo.2014.00086
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. science 284(5411):143–147
Rothrauff BB et al (2019) Stem cell therapy for musculoskeletal diseases. In:Atala A et al (ed) Principles of Regenerative Medicine, 3rd edn. Elsevier (Academic Press), London, United Kingdom, pp 953–970. https://doi.org/10.1016/C2015-0-02433-9
Schmelzer E (2019) Hepatic progenitors of the fetal liver: interactions with hematopoietic stem cells. Differentiation. https://doi.org/10.1016/j.diff.2019.02.005
Chen W et al (2018) Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J Tissue Eng Regen Med 12(1):191–203
Sackstein R et al (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14(2):181
Laird DJ, von Andrian UH, Wagers AJ (2008) Stem cell trafficking in tissue development, growth, and disease. Cell 132(4):612–630
Boroujeni ME, Gardaneh M (2017) Umbilical cord: an unlimited source of cells differentiable towards dopaminergic neurons. Neural Regen Res 12(7):1186
Bellin MD et al (2012) Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 12(6):1576–1583
Thakkar UG et al (2015) Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow–derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 17(7):940–947
Wang Z-X et al (2015) Clinical efficacy of autologous stem cell transplantation for the treatment of patients with type 2 diabetes mellitus: a meta-analysis. Cytotherapy 17(7):956–968
Moshkovska T, Mayberry J (2005) It is time to test low level laser therapy in Great Britain. Postgrad Med J 81(957):436–441
Hendudari F et al (2016) Combined effects of low-level laser therapy and human bone marrow mesenchymal stem cell conditioned medium on viability of human dermal fibroblasts cultured in a high-glucose medium. Lasers Med Sci 31(4):749–757
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16(1):4
Gavish L et al (2008) Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg Med 40(5):371
Giannelli M et al (2013) Photoactivation of bone marrow mesenchymal stromal cells with diode laser: effects and mechanisms of action. J Cell Physiol 228(1):172–181
Rizzi CF et al (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-κB signaling pathway in traumatized muscle. Lasers Surg Med 38(7):704–713
Gasparyan L, Brill G, Makela A (2004) Influence of low level laser radiation on migration of stem cells. Laser Florence 2004:1–7
Ayuk SM, Abrahamse H, Houreld NN (2018) Photobiomodulation alters matrix protein activity in stressed fibroblast cells in vitro. J Biophotonics 11(3):e201700127
Ginani F, Soares DM, Barboza CAG (2015) Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med Sci 30(8):2189–2194
Tsuka Y et al (2019) Effects of Nd: YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Lasers Med Sci 34(1):55–60
Eichler M et al (2007) Red light-induced redox reactions in cells observed with TEMPO. Photomed Laser Surg 25(3):170–174
Yin K et al (2017) Low-level laser effect on proliferation, migration, and antiapoptosis of mesenchymal stem cells. Stem Cells Dev 26(10):762–775
Li X et al (2014) Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 34(3):695–704
Esmaeelinejad M et al (2014) The effects of low-level laser irradiation on cellular viability and proliferation of human skin fibroblasts cultured in high glucose mediums. Lasers Med Sci 29(1):121–129
Piryaei A et al (2015) Ultrastructural maturation of human bone marrow mesenchymal stem cells-derived cardiomyocytes under alternative induction of 5-azacytidine. Cell Biol Int 39(5):519–530
Bullwinkel J et al (2006) Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol 206(3):624–635
Bruno S, Darzynkiewicz Z (1992) Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif 25(1):31–40
Kyrylkova K et al (2012) Detection of apoptosis by TUNEL assay. In: Odontogenesis: Methods and Protocols, pp 41–47
Jin P et al. (2010) Streptozotocin-induced diabetic rat–derived bone marrow mesenchymal stem cells have impaired abilities in proliferation, paracrine, antiapoptosis, and myogenic differentiation. Transplant Proc 42(7):2745–2752
Cianfarani F et al (2013) Diabetes impairs adipose tissue–derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen 21(4):545–553
Zhao Y-F et al (2013) Osteogenic potential of bone marrow stromal cells derived from streptozotocin-induced diabetic rats. Int J Mol Med 31(3):614–620
Khan M et al (2010) Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev 20(1):67–75
Yaochite JNU et al (2016) Multipotent mesenchymal stromal cells from patients with newly diagnosed type 1 diabetes mellitus exhibit preserved in vitro and in vivo immunomodulatory properties. Stem Cell Res Ther 7(1):14
Kushibiki T et al (2015) Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int. https://doi.org/10.1155/2015/974864
Fallahnezhad S et al (2016) Low-level laser therapy with helium-neon laser improved viability of osteoporotic bone marrow-derived mesenchymal stem cells from ovariectomy-induced osteoporotic rats. J Biomed Opt 21(9):98002
Cavalcanti MFXB et al (2015) Evaluation of the proliferative effects induced by low-level laser therapy in bone marrow stem cell culture. Photomed Laser Surg 33(12):610–616
Li W-T, Leu Y-C, Wu J-L (2010) Red-light light-emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Photomed Laser Surg 28(S1):S-157–S-165
Huang Y-Y et al (2009) Biphasic dose response in low level light therapy. Dose Response 7(4):358-383
Soares DM et al (2015) Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med Sci 30(3):1171–1174
Soleimani M et al (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—an in vitro study. Lasers Med Sci 27(2):423–430
Filip S, Mokrý J, Hruska I (2003) Adult stem cells and their importance in cell therapy. Folia Biol 49(1):9–14
Acknowledgments
The present article is based on the thesis by Fatemeh Zare at the School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Registration No. M64).
Funding
This study was supported by Regenerative Medicine and Stem Cell Research Network, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 7803).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zare, F., Bayat, M., Aliaghaei, A. et al. Photobiomodulation therapy compensate the impairments of diabetic bone marrow mesenchymal stem cells. Lasers Med Sci 35, 547–556 (2020). https://doi.org/10.1007/s10103-019-02844-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02844-y