Abstract
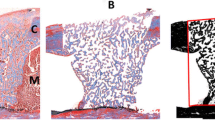
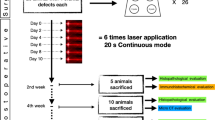
This study aimed to evaluate, through histomorphometric analysis, the bone repair process in the tibia of rats treated with zoledronic acid and submitted to 808-nm low-level laser therapy (LLLT) by using arsenide aluminum gallium laser. For this purpose, 20 rats were used and distributed according to treatment: group 1—saline administration; group 2—treated with LLLT; group 3—treated with zoledronic acid; and group 4—treated with zoledronic acid and LLLT. The zoledronic acid was administered at a dose of 0.035 mg/kg every 2 weeks for 8 weeks. Subsequently, bone defects of 2 mm were prepared in the tibias of all groups. The bone defects in groups 2 and 4 were irradiated with LLLT in the immediate post-operative period. After 14 and 28 days of application, the animals were submitted and euthanized for histomorphometric analysis. The results were submitted to statistical analysis (α = 5%), and the intragroup comparison was performed using the t test. On the other hand, for intergroup comparison, the ANOVA test was performed, and to the groups presenting statistically significant difference, the Student-Newman-Keuls test was used. In intergroup comparison, group 1 (mean ± SD= 45.2 ± 18.56%) showed a lower bone formation compared with groups 2 (64.13 ± 3.51%) (p = 0.358) and 4 (15.2 ± 78.22%) (p = 0.049), at the 14-day period. Group 3 (20.99 ± 7.42%) also presented a lower amount of neoformed bone tissue, with statistically significant difference when compared with groups 1 (p = 0.002), 2, and 4 (p ≤ 0,001). After 28 days, group 1 presented a lower amount of neoformed bone tissue compared with the other groups, with p = 0.020. Thus, it was concluded that LLLT associated with zoledronic acid is effective for stimulating bone formation in surgically created defects in rats, at the periods studied.





Similar content being viewed by others
References
Cheng A, Daly CG, Logan RM, Stein B, Goss AN (2009) Alveolar bone and the bisphosphonates. Aust Dent J 54:S51–S61
Yamashita J, Koi K, Yang DY, McCauley LK (2011) Effect of zoledronate on oral wound healing in rats. Clin Cancer Res 17:1405–1414
Borromeo GL, Tsao CE, Darby IB, Ebeling PR (2011) A review of the clinical implications of bisphosphonates in dentistry. Aust Dent J 56:2–9
Palacio EP, Müller SS, Sardenberg T, Mizobuchi RR, Galbiatti JA, Durigan A Jr, Savarese A, Ortolan EV (2012) Detecting early biomechanical effects of zoledronic acid on femurs of osteoporotic female rats. J Osteoporos 2012:162802
Cardemil C, Omar OM, Norlindh B, Wexell CL, Thomsen P (2013) The effects of a systemic single dose of zoledronic acid on post-implantation bone remodelling and inflammation in an ovariectomised rat model. Biomaterials 34:1546–1561
Garcia VG, Conceição JM, Fernandes LA, Almeida JM, Nagata MJH, Bosco AF, Theodoro LH (2013) Effects of LLLT in combination with bisphosphonate on bone healing in critical size defects: a histological and histometric study in rat calvaria. Lasers Med Sci 28:407–414
Wynn RL (2005) Bisphosphonates, hypercalcemia of malignancy, and osteonecrosis of the jaw. Gen Dent 53:392–395
Ebrahimi T, Moslemi N, Rokn AR, Heidari M, Nokhbatolfoghahaie H, Fekrazad R (2012) The influence of low-intensity laser therapy on bone healing. J Dent 9:238–248
Jakse N, Payer M, Tangl S, Berghold A, Kirmeier R, Lorenzoni M (2007) Influence of low- level laser treatment on bone regeneration and osseointegration of dental implants following sinus augmentation. An experimental study on sheep. Clin Oral Implants Res 18:517–524
Nascimento SB, Cardoso CA, Ribeiro TP, Almeida JD, Albertini R, Munin E, Arissawa EA (2010) Effect of low level laser therapy and calcitonin on bone repair in castiated rats: a densitometric study. Photomed Laser Surg 28:45–49
Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I (2010) Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 25:1337–1349
Yu YY, Lieu S, Hu D, Miclau T, Colnot C (2012) Site specific effects of zoledronic acid during tibial and mandibular fracture repair. PLoS One 7:e31771–e31780
Botell M (2001) Osteoporosis en la menopausia, prevención y estratégias terapêuticas atuales. Rev Cuba Obstet Ginecol 27:199–204
Amanat N, McDonald M, Godfrey C, Bilston L, Little D (2007) Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 22:867–876
Camacho AF, López JP, Vicente HA (2013) Short-term effect of zoledronic acid upon fracture resistance of the mandibular condyle and femoral head in an animal model. Med Oral Patol Oral Cir Bucal 18:e421–e426
Back DA, Pauly S, Rommel L, Hass NP, Schmidmaier G, Wildemann B, Greiner SH (2012) Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord 13:42–50
Yaman F, Agaçayak S, Atilgan S, Benliday E, Ucan MC, Erol B, Kaya B, Gunay A, Guven S (2012) Effects of systemic zoledronic acid administration on osseointegration of hydroxyapatite-coated and resorbable blast material surface implants in rabbit models. Int J Oral Maxillofac Implants 27:1443–1447
Okamoto Y, Hirota M, Monden Y, Murata S, Koyama C, Mitsudo K, Iwai T, Ishikawa Y, Tohnai I (2013) High-dose zoledronic acid narrows the periodontal space in rats. Int J Oral Maxillofac Surg 42:627–631
Çankaya M, Senel ÇF, Duman KM, Muci E, Dayisoylu EH, Balaban F (2013) The effects of chronic zoledronate usage on the jaw and long bones evaluated using RANKL and osteoprotegerin levels in an animal model. Int J Oral Maxillofac Surg 42:1134–1139
Ribeiro DA, Matsumoto MA (2009) Low-level laser therapy improves bone repair in rats treated with anti-inflammatory drugs. J Oral Rehabil 35:925–933
Matsumoto MA, Ferino RV, Monteleone GF, Ribeiro DA (2009) Low-level laser therapy modulates cycloxygenase-2 expression during bone repair in rats. Laser Med Sci 24:195–201
Fáravo-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, Araújo HSSA, Renno ACM (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Fernandes KR, Ribeiro DA, Rodrigues NC, Tim C, Santos AA, Parizotto NA, Araujo HS, Driusso P, Rennó ACM (2013) Effects of low-level laser therapy on the expression. Of osteogenic genes related in the initial stages of bone defects in rats. J Biomed Opt 18:038002
Barbosa D, Villaverde AGJB, Arisawa EAL, Souza RA (2014) Laser therapy in bone repair in rats: analysis of bone optical density. Acta Ortop Bras 22:71–74
Marques L, Holgado LA, Francischone LA, Ximenez JPB, Okamoto R, Kinoshita (2015) A new LLLT protocol to speed up the bone healing process—histometric and immunohistochemical analysis in rat calvarial bone defect. Lasers Med Sci 30:1225–1230
Sener I, Bereket C, Kosker H, Turer A, Tek M, Kaplan S (2013) The effects of zoledronic acid on mandibular fracture healing in an osteoporotic model: a stereological study. J Craniofac Surg 24:1221Y1224
Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S (2009) Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab 27:663–672
Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ (2010) Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis 16:674–685
Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T (2010) Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28:165–175
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: PLS, LMS, JLG, BLCI, LMLR; performed the experiments: VCB, EJG, JLG, PLS; analyzed the data: WO, BLCI, LMLR, PLS; contributed reagents/materials/analysis tools: WO, VCB, EJG, LMS; wrote the paper: PLS, LMS, JLG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (attached supplementary materials—protocol number 006/13).
Informed consent
The authors declare that it was not necessary to have informed consent since the research was not conducted in humans.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buchignani, V.C., Germano, E.J., dos Santos, L.M. et al. Effect of low-level laser therapy and zoledronic acid on bone repair process. Lasers Med Sci 34, 1081–1088 (2019). https://doi.org/10.1007/s10103-019-02810-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02810-8