Abstract
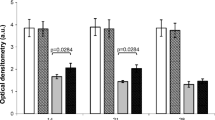
This study evaluates possible changes in weight and the secretory patterns of the thyroid and parathyroid glands irradiated with a 780-nm light-emitting diode (LED) source under conditions allowing their identification in an animal model. The use of near-infrared light sources to detect the parathyroid glands has been a subject of research due to the benefit provided to patients undergoing thyroid and parathyroid surgery. However, few studies have explored possible changes in weight and the secretory patterns of the glands when subjected to light stimulation. Thirty-two male Wistar rats were divided into two groups and subjected to cervical dissection and irradiation of the thyroid-parathyroid region under general anesthesia. The animals in group 1 (irradiated) were exposed to a 780-nm LED light beam for 180 s (dose 1.37 J/cm2), whereas the animals in group 2 (control) were not irradiated. Blood samples were collected pre-exposure, 7 min after exposure, and 30 and 60 days after exposure to measure calcium, parathyroid hormone (PTH), triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH) levels in both groups. Weight variations between the evaluation periods were also analyzed. Parametric statistics were used (Student’s t test and ANOVA) after assuming normal distribution of continuous variables with the Shapiro-Wilk test. No significant variation was observed in any of the analyzed parameters pre- and postexposure. A significant increase in the calcium level was observed at 30 days in the irradiated group compared with that in the control group (11.156 ± 1.31 mg/dl vs 10.300 ± 0.30 mg/dl; df = 30 p < 0.03) but this change was no longer observed at 60 days (9.925 ± 0.23 mg/dl vs 10.014 ± 0.18 mg/dl; df = 14 p = 0.21). Irradiated rats gained less weight in the first 30 days after surgery (39.647 ± 32.39 vs 55.917 ± 22.61; df = 30 p = 0.146) and at 60 days (84.000 ± 27.36 vs 84.571 ± 5.62; df = 14 p = 0.957), no differences were observed between the two groups. No significant variations in weight development or changes in the secretory patterns of the thyroid and parathyroid glands exposed to near-infrared stimulation were observed, confirming the safety of this light source in the identification of the parathyroid glands.






Similar content being viewed by others
References
McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT (2013) A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154:1371–1377
Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R (2016) Cutting edge in thyroid surgery: autofluorescence of parathyroid glands. J Am Coll Surg 223:374–380
Ladurner R, Sommerey S, Arabi NA, Hallfeldt KKJ, Stepp H, Gallwas JKS (2017) Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 31:3140–3145
Kim SW, Lee HS, Ahn YC, Park CW, Jeon SW, Kim CH, Ko JB, Oak C, Kim Y, Lee KD (2018) Near-infrared autofluorescence image-guided parathyroid gland mapping in thyroidectomy. J Am Coll Surg 226:165–172
Akerström G, Malmaeus J, Bergström R (1984) Surgical anatomy of human parathyroid glands. Surgery 95:14–21
Policeni BA, Smoker WR, Reede DL (2012) Anatomy and embryology of the thyroid and parathyroid glands. Semin Ultrasound CT MR 33:104–114
Fancy T, Gallagher D 3rd, Hornig JD (2010) Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin N Am 43:221–227 vii
Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, Shah JP, Fremgen AM, Stewart AK, Hölzer S (2000) Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German thyroid cancer study group. An American College of Surgeons Commission on cancer patient care evaluation study. Cancer 89:202–217
Pattou F, Combemale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C (1998) Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 22:718–724
Rafferty MA, Goldstein DP, Rotstein L, Asa SL, Panzarella T, Gullane P, Gilbert RW, Brown DH, Irish JC (2007) Completion thyroidectomy versus total thyroidectomy: is there a difference in complication rates? An analysis of 350 patients. J Am Coll Surg 205:602–607
Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R (1998) The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg 228:320–330
Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16:067012
McWade MA, Mahadevan-Jansen A, Jansen ED, Galloway RL, McDonald WH, Broome JT (2016) Development of an intraoperative tool to detect parathyroid gland autofluorescence. Vanderbilt University, Tennessee
De Leeuw F, Breuskin I, Abbaci M, Casiraghi O, Mirghani H, Ben Lakhdar A, Laplace-Builhé C, Hartl D (2016) Intraoperative near-infrared imaging for parathyroid gland identification by auto-fluorescence: a feasibility study. World J Surg 40:2131–2138
van der Vorst JR, Schaafsma BE, Verbeek FP, Swijnenburg RJ, Tummers QR, Hutteman M, Hamming JF, Kievit J, Frangioni JV, van de Velde CJ, Vahrmeijer AL (2014) Intraoperative near-infrared fluorescence imaging of parathyroid adenomas with use of low-dose methylene blue. Head Neck 36:853–858
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, Berlin
Azevedo LH, Aranha AC, Stolf SF, Eduardo Cde P, Vieira MM (2005) Evaluation of low intensity laser effects on the thyroid gland of male mice. Photomed Laser Surg 23:567–570
Mohammed IFR, Al-Azawi TS, AL-Mustawfi NS (2011) Effect of laser treatment on thyroid gland hormones in female rabbits. Iraqi J Vet Sci 25:61–64
Fronza B, Somacal T, Mayer L, de Moraes JF, de Oliveira MG, Weber JB (2013) Assessment of the systemic effects of low-level laser therapy (LLLT) on thyroid hormone function in a rabbit model. Int J Oral Maxillofac Surg 42:26–30
Lerma E, Hevia A, Rodrigo P, Gonzalez-Campora R, Armas JR, Galera H (1991) The effect of HeNe laser radiation on the thyroid gland of the rat. Int J Exp Pathol 72:379–385
Weber JB, Mayer L, Cenci RA, Baraldi CE, Ponzoni D, Gerhardt de Oliveira M (2014) Effect of three different protocols of low-level laser therapy on thyroid hormone production after dental implant placement in an experimental rabbit model. Photomed Laser Surg 32:612–617
Stolik S, Delgado JA, Pérez A, Anasagasti L (2000) Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J Photochem Photobiol B 57:90–93
Kim SW, Lee HS, Lee KD (2017) Intraoperative real-time localization of parathyroid gland with near infrared fluorescence imaging. Gland Surg 6:516–524
Kittel B, Ernst H, Kamino K (1996) Anatomy, histology, ultrastructure, parathyroid, rat, in endocrine system. Springer, Berlin Heidelberg, pp 330–333
Acknowledgments
This study would not have been possible without the collaboration of Dr. Joaquim Machado Caetano, Dr. Sofia Jorge, and the technicians at Laboratório Affidea who conducted the laboratory analyses of all the animals. At the UBI vivarium, we received indispensable help from the technicians Maria João and Maria José with blood collection and surgery as well as with the care and maintenance of the animals.
We had also the collaboration of Professors Graça Baltazar and Manuel Lemos and Drs. Maria do Rosário Custódio, António Gomes and Sílvia Silva.
We express our gratitude to all who helped with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethical commission of the Faculdade de Ciências da Saúde da Universidade da Beira Interior- Portugal (CE-FCS-2014-022) and by Direcção-geral de Agricultura e Veterinária (0421/000/000/2018), and performed respecting the principles of European Directive 2010/63/EU.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serra, C., Silveira, L. Near-infrared irradiation of the thyroid area: effects on weight development and thyroid and parathyroid secretory patterns. Lasers Med Sci 35, 107–114 (2020). https://doi.org/10.1007/s10103-019-02800-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02800-w