Abstract
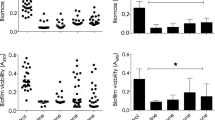
Infections caused by Acinetobacter baumannii have become a challenge for healthcare professionals because of the rapid increase in Gram-negative bacteria resistant to carbapenem antibiotics. The objective of this study was to evaluate the effect of antimicrobial photodynamic therapy (aPDT) against different strains of A. baumannii isolated from patients with infectious process and hospitalized at the intensive care unit of the hospitals of São Jose dos Campos, São Paulo. These isolates were obtained from the Valeclin Clinical Analysis Laboratory (SP, Brazil) and were tested for susceptibility to the carbapenems imipenem and meropenem by determination of the minimal inhibitory concentration (MIC) using the broth microdilution method. The strains susceptible and resistant to these antibiotics were submitted to aPDT using methylene blue and a low-level laser with a wavelength of 660 nm and fluence of 39.5 J/cm2 (energy of 15 J and time of 428 s). The number of colony-forming units (CFU/mL) was analyzed by ANOVA and the Tukey test. The laboratory of origin of the clinical isolates identified 1.54% of 13,715 strains tested over a period of 8 months as A. baumannii. Among the A. baumannii isolates, 58% were resistant to carbapenems by the disk diffusion test. Susceptible isolates exhibited MIC of 0.5 to 1 μg/mL and resistant isolates of 64 to > 128 μg/mL. PDT reduced the number of A. baumannii cells for all isolates tested, with this reduction ranging from 63 to 88% for susceptible isolates and from 26 to 97% for resistant isolates. The percentage of viability was dependent on the strain analyzed. In conclusion, these data indicate that PDT could be an alternative strategy for the control of infections caused by carbapenem-resistant A. baumannii.



Similar content being viewed by others
References
Cisneros JM, Rodríguez-Baño J (2002) Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect 8:687–693
Karampatakis T, Antachopoulos C, Tsakris A et al (2017) Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in Greece: an extended review (2000-2015). Future Microbiol 12:801–815
Howard A, O' Donoghue M, Feeney A, Sleator RD (2012). Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250
Lee CR, Lee JH, Park M et al (2017) Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 13:55
Lemos EV, Ia Hoz FP, Einarson TR et al (2014) Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 20(5):416–423
Perez F, Hujer AM, Hujer KM et al (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484
Kuo SC, Chang SC, Wang HY et al (2012) Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 12:200
Izadpanah M, Khalili H (2015) Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract 4:105–114
Winter JS, Santos RP, Azambuja AZ et al (2013) Microbiologic isolates and risk factors associated with antimicrobial resistance in patients admitted to the intensive care unit in a tertiary care hospital. Am J Infect Control 41:846–848
Gales AC, Castanheira M, Jones RN et al (2012) Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008-2010). Diagn Microbiol Infect Dis 73:354–360
Sperandio FF, Huang YY, Hamblin MR (2013) Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov 8(2):108–120
Ragàs X, Dai T, Tegos GP et al (2010) Photodynamic inactivation of Acinetobacter baumannii using phenothiazinium dyes: in vitro and in vivo studies. Lasers Surg Med 42(5):384–390
Maisch T, Hackbarth S, Regensburger J et al (2011) Photodynamic inactivation of multi-resistant bacteria (PIB)—a new approach to treat superficial infections in the 21st century. J Dtsch Dermatol Ges 9(5):360–366
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chem 42:13–28
Demidova TN, Hamblin MR (2004) Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol 17:245–254
Dai T, Tegos GP, Lu Z et al (2009) Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother 53(9):3929–3934
Gaynes R, Edwards JR (2005) Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41(6):848–854
Johnson EN, Burns TC, Hayda RA et al (2007) Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis 45:409–415
Sebeny PJ, Riddle MS, Petersen K (2008) Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis 47:444–449
Moradi J, Hashemi FB, Bahador A (2015) Antibiotic resistance of Acinetobacter baumannii in Iran: a systemic review of the published literature. Osong Public Health Res Perspect 6(2):79–86
Zhang Y, Zhu Y, Gupta A et al (2014) Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis 209:1963–1971
(2013) Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing [M100-S23]. Clinical and Laboratory Standards Institute, Wayne, PA, USA
Souza LC, Brito PR, de Oliveira JC et al (2010) Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J Endod 36:292–296
Liu JW, Ko WC, Huang CH et al (2012) Agreement assessment of tigecycline susceptibilities determined by the disk diffusion and broth microdilution methods among commonly encountered resistant bacterial isolates: results from the tigecycline in vitro surveillance in Taiwan (TIST) Study, 2008 to 2010. Antimicrob Agents Chemother 56(3):1414–1417
Jiang H, Hu H, Ren H et al (2016) Retrospective data about the catheter-related complications and management in massive bus burn casualties. J Vasc Access 17(4):353–359
Tien N, You BJ, Chang HL et al (2012) Comparison of genospecies and antimicrobial resistance profiles of isolates in the Acinetobacter calcoaceticus-Acinetobacter baumannii complex from various clinical specimens. Antimicrob Agents Chemother 56:6267–6271
Garcez AS, Núñez SC, Azambuja N Jr et al (2013) Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic analysis. Photomed Laser Surg 31:519–525
Misba L, Zaidi S, Khan AU (2017) A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilms. Photodiagn Photodyn Ther 18:24–33
de Figueiredo Freitas LS, Rossoni RD, Jorge AO et al (2017) Repeated applications of photodynamic therapy on Candida glabrata biofilms formed in acrylic resin polymerized. Lasers Med Sci 32(3):549–555
Altun HU, Yagci S, Bulut C et al (2014) Antimicrobial susceptibilities of clinical Acinetobacter baumanniiisolates with different genotypes. Jundishapur J Microbiol 7:e13347
Izadpou F, Ranjbari N, Aramesh MR et al (2016) An investigation of antibacterial resistance patterns among Acinetobacter baumannii and Pseudononas aeruginosa isolates collected from intensive care units of a university-affiliated hospital in Ahvaz, Iran. Jundishapur J Microbiol 9:e35624
Clark NM, Zhanel GG, Lynch JP (2016) Emergence of antimicrobial resistance among Acinetobacter species: a global threat. Curr Opinion Crit Care 22:491–499
Maraki S, Mantadakis E, Mavromanolaki VE et al (2016) A 5-year surveillance study on antimicrobial resistance of Acinetobacter baumannii clinical isolates from a tertiary greek hospital. Infect Chemother 48:190–198
Wong D, Nielsen TB, Bonomo RA et al (2017) Clinical and pathophysiological overview of Acintetobacter infections: a century of challenges. Clin Microbiol Res 30:409–447
Wang X, Tian F, Soni SS et al (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 6:30540
Rupel K, Zupin L, Colliva A et al (2018) Photobiomodulation at multiple wavelengths differentially modulates oxidative stress in vitro and in vivo. Oxidative Med Cell Longev 2018:6510159
Chung H, Dai T, Sharma SK et al (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533
Gupta A, Avci P, Sadasivam M et al (2012) Shining light on nanotechnology to help repair and regeneration. Biotechnol Adv 31(5):607–631
Avci P, Gupta A, Sadasivam M et al (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 32(1):41–52
Peplow PV, Chung TY, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29(5):285–304
Kuffler DP (2016) Photobiomodulation in promoting wound healing: a review. Regen Med 11(1):107–122
Wang X, Reddy DD, Nalawade SS et al (2018) Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics 5(1):011004
Dos Santos JD, de Alvarenga JA, Rossoni RD et al (2017) Immunomodulatory effect of photodynamic therapy in Galleria mellonella infected with Porphyromonas gingivalis. Microb Pathog 110:507–511
Role of funding source
This study was supported by the São Paulo Council of Research - FAPESP, Brazil (Grant 2014/03937-6).
M.M.M received a doctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
P.P.B received a doctoral fellowship from FAPESP, Brazil (Grant 2012/15250-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee (Process: 24409813.9.0000.0077) of the Institute of Science and Technology of Univ. Estadual Paulista (ICT/UNESP).
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marcolan De Mello, M., De Barros, P.P., de Cassia Bernardes, R. et al. Antimicrobial photodynamic therapy against clinical isolates of carbapenem-susceptible and carbapenem-resistant Acinetobacter baumannii. Lasers Med Sci 34, 1755–1761 (2019). https://doi.org/10.1007/s10103-019-02773-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02773-w