Abstract
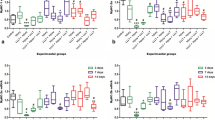
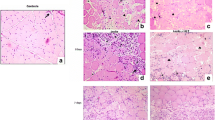
The aim of this study was to determine the effects of gallium arsenide (GaAs) laser on IGF-I, MyoD, MAFbx, and TNF-α gene expression during the intermediate phase of muscle regeneration after cryoinjury 21 Wistar rats were divided into three groups (n = 7 per group): untreated with no injury (control group), cryoinjury without GaAs (injured group), and cryoinjury with GaAs (GaAs-injured group). The cryoinjury was induced in the central region of the tibialis anterior muscle (TA). The region injured was irradiated once a day during 14 days using GaAs laser (904 nm; spot size 0.035 cm2, output power 50 mW; energy density 69 J cm−2; exposure time 4 s per point; final energy 4.8 J). Twenty-four hours after the last application, the right and left TA muscles were collected for histological (collagen content) and molecular (gene expression of IGF-I, MyoD, MAFbx, and TNF-α) analyses, respectively. Data were analyzed using one-way ANOVA at P < 0.05. There were no significant (P > 0.05) differences in collagen density and IGF-I gene expression in all experimental groups. There were similar (P < 0.05) decreases in MAFbx and TNF-α gene expression in the injured and GaAs-injured groups, compared to control group. The MyoD gene expression increased (P = 0.008) in the GaAs-injured group, but not in the injured group (P = 0.338), compared to control group. GaAs laser therapy had a positive effect on MyoD gene expression, but not IGF-I, MAFbx, and TNF-α, during intermediary phases (14 days post-injury) of muscle repair.



Similar content being viewed by others
References
Alonso JM, Edouard P, Fischetto G, Adams B, Depiesse F, Mountjoy M (2012) Determination of future prevention strategies in elite track and field: analysis of Daegu 2011 IAAF Championships injuries and illnesses surveillance. Br J Sports Med 46:505–514
Freckleton G, Pizzari T (2013) Risk factors for ham string muscle strain injury in sport: a systematic review and meta-analysis. Br J Sports Med 47:351–358
Winkler T, Roth PV, Matziolis G, Schumann MR, Hahn S, Strube P, Stoltenburg-Didinger G, Perka C, Duda GN, Tohtz SV (2011) Time course of skeletal muscle regeneration after severe trauma. Acta Orthop 82:102–111
Eksrean DJ, Hägglund MW, Aldenan DM (2011) Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 39:1226–1232
Rodrigues NC, Brunelli R, de Araujo HSS, Parizotto NA, Renno ACM (2013) Low-level laser therapy (LLLT) (660 nm) alters gene expression during muscle healing in rats. J Photochem Photobiol B 120:29–35
Alves AN, Fernandes KPS, Melo CAV, Yamaguchi RY, França CM, Teixeira DF, Bussadori SK, Nunes FD, Mesquita-Ferrari RA (2014) Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med Sci 29:813–821
Freitas CEA, Bertaglia RS, Junior IJV, Mareco EA, Salomão RAS, de Paula TG, Nai GA, Carvalho RF, Francis Lopes Pacagnelli FL, Dal-Pai-Silva M (2015) High final energy of low-level gallium arsenide laser therapy enhances skeletal muscle recovery without a positive effect on collagen remodeling. Photochem Photobiol 91:957–965
Shefer G, Nadav Ben-Dov N, Orna Halevy O, Oron U (2008) Primary myogenic cells see the light: improved survival of transplanted myogenic cells following low energy laser irradiation. Lasers Surg Med 40:38–45
Assis L, Moretti AIS, Abrahão TB, de Souza HP, Hamblin MR, Parizotto NA (2013) Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 28:947–955
Eduardo FP, Dumehnert Monezi TA (2007) Cultured epithelial cells response to phototherapy with low intensity laser. Lasers Surg Med 39:365–372
Alves AN, Fernandes KP, Deana AM, Bussadori SK, Mesquita-Ferrari RA (2014) Effects of low-level laser therapy on skeletal muscle repair: a systematic review. Am J Phys Med Rehabil 93:1073–1085
da Silva JP, Da Silva MA, Almeida AP, Lombardi Junior I, Matos AP (2010) Laser therapy in the tissue repair process: a literature review. Photomed Laser Surg 28:17–21
Dawood MS, AL Salihi AR, Qasim AW (2013) Laser therapy of muscle injuries. Lasers Med Sci 28:735–742
Dourado DM, Matias R, Almeida MF, de Paula KR, Vieira RP, Oliveira LVF, Carvalho PTC (2008) The effects of low-level laser on muscle damage caused by Bothrops neuwiedi venom. J Venom Anim Toxins Incl Trop Dis 14:423–434
Silva LH, Silva MT, Gutierrez RM, Conte TC, Toledo CA, Aoki MS, Liebano RE, Miyabara EH (2012) GaAs 904-nm laser irradiation improves myofiber mass recovery during regeneration of skeletal muscle previously damaged by crotoxin. Lasers Med Sci 27:993–1000
Pissulin CNA, Fernandes AAH, Orellana AMS, Silva RCR E, Matheus SMM (2017) Low-level laser therapy (LLLT) accelerates the sternomastoid muscle regeneration process after myonecrosis due to bupivacaine. J Photochem Photobiol B 168:30–39
Oliveira NML, Parizzotto NA, Tania SF (1999) GaAs (904-nm) laser radiation does not affect muscle regeneration in mouse skeletal muscle. Lasers Surg Med 25:13–21
Alves AN, Ribeiro BG, Fernandes KP, Souza NH, Rocha LA, Nunes FD, Bussadori SK, Mesquita-Ferrari RA (2016) Comparative effects of low-level laser therapy pre- and post-injury on mRNA expression of MyoD, myogenin, and IL-6 during the skeletal muscle repair. Lasers Med Sci 31(4):679–685
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–340
Karalaki M, Fili S, Philippou A, Koutsilieris M (2009) Muscle regeneration: cellular and molecular events. In Vivo 23:779–796
Ten Broek RW, Grefte S, Den Hoff JWV (2010) Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224:7–16
Assis L, Moretti AI, Abrahão TB, Cury V, Souza HP, Hamblin MR, Parizotto NI (2012) Low-level laser therapy (808 nm) angiograms inflammatory response and oxidative stress in rat tibial is anterior muscle after cryolesion. Lasers Surg Med 44:726–735
Morrone G, Guzzardella GA, Orienti LL, Giavaresi G, Fini M, Rocca M, Torricelli P, Martini L, Giardino R (1998) Muscular trauma treated with a Ga-Al-As diode laser: in vivo experimental study. Lasers Med Sci 13:293–298
Calvi EN, Nahas FX, Barbosa MV, Calil JA, Ihara SS, Silva SM, Franco MF, Ferreira LM (2012) An experimental model for the study of collagen fibers in skeletal muscle. Acta Cir Bras 27:681–686
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta C(T) method. Methods 25:402–408
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR by geometric averaging of multiple internal control genes. Genome Biol 334:0034
Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M (2005) Muscle injuries: biology and treatment. Am J Sports Med 33:745–764
Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musarò A (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21:1393–1402
Musarò A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N (2004) Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A 101:1206–1210
Zanou N, Gailly P (2013) Skeletal muscle hypertrophy and regeneration: the interplay between myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci 70:4117–4130
Xu Q, Wu Z (2000) The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates Myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem 275:36750–36757
Hill M, Goldspink G (2003) Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418
Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L (2002) Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics 11:263–272
Chargé SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
World Association of Laser Therapy (WALT) (2006) Consensus agreement on the design and conduct of clinical studies with low level laser therapy and light therapy for musculoskeletal pain and disorders. Photomed Laser Surg 24:761–762
Pissulin CN, de Souza Castro PA, Codina F, Pinto CG, Vechetti-Junior IJ, Matheus SM (2017) GaAs laser therapy reestablishes the morphology of the NMJ and nAChRs after injury due to bupivacaine. J Photochem Photobiol B 167:256–263
Rizzi CF, Mauriz JL, Correa DSF, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, Lez-Gallego JG (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kB signaling pathway in traumatized muscle. Lasers Surg Med 38:704–713
Silveira PCL, Silva LA, Pinho CA, Souza PS, Ronsani MM, Scheffer D, Pinho RA (2013) Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci 28:431–436
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA (2005) Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 280:2847–2856
Okada A, Ono Y, Nagatomi R, Kishimoto KN, Itoi E (2008) Decreased muscle atrophy F-box (MAFbx) expression in regenerating muscle after muscle-damaging exercise. Muscle Nerve 38:1246–1253
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98:1745–1752
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103:1744–1751
Szalay K, Duda E (1997) TNF inhibits myogenesis and downregulates the expression of myogenic regulatory factors myoD and myogenin. Eur J Cell Biol 74:391–398
Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2004) Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18:227–237
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB (1998) Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor alpha. FASEB J 12:871–880
Baptista J, Martins MD, Pavesi VCS, Bussadori SK, Fernandes KPS, Junior DSP, Mesquita-Ferrari RA (2010) Influence of laser photo biomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29:1–7
Funding
We thank the Institutional Program of Scientific Initiation Scholarships (PIBIC) from the National Council for Scientific and Technological Development (CNPq) for financial support (Process No. 146045/2013–0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures were approved by the Ethics Research Committee of the University of Western São Paulo (UNOESTE), Presidente Prudente, São Paulo, Brazil (Protocol No. 713).
Rights and permissions
About this article
Cite this article
Santos, C.P., Aguiar, A.F., Giometti, I.C. et al. High final energy of gallium arsenide laser increases MyoD gene expression during the intermediate phase of muscle regeneration after cryoinjury in rats. Lasers Med Sci 33, 843–850 (2018). https://doi.org/10.1007/s10103-018-2439-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2439-3