Abstract
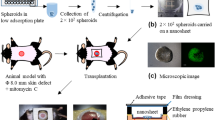
Skin flap grafting is a form of transplantation widely used in plastic surgery. However, ischemia/reperfusion injury is the main factor which reduces the survival rate of flaps following grafting. We investigated whether photobiomodulation (PBM) precondition prior to human adipose-derived stromal cell (hASC) spheroid (PBM-spheroid) transplantation improved skin tissue functional recovery by the stimulation of angiogenesis and tissue regeneration in skin flap of mice. The LED had an emission wavelength peaked at 660 ± 20 nm (6 J/cm2, 10 mW/cm2). The expression of angiogenic growth factors in PBM-spheroid hASCs was much greater than that of not-PBM-treated spheroid or monolayer-cultured hASCs. From immunochemical staining analysis, the hASCs of PBM-spheroid were CD31+, KDR+, and CD34+, whereas monolayer-cultured hASCs were negative for these markers. To evaluate the therapeutic effect of hASC PBM-spheroid in vivo, PBS, monolayer-cultured hASCs, and not-PBM-spheroid were transplanted into a skin flap model. The animals were observed for 14 days. The PBM-spheroid hASCs transplanted into the skin flap ischemia differentiated into endothelial cells and remained differentiated. Transplantation of PBM-spheroid hASCs into the skin flap ischemia significantly elevated the density of vascular formations through angiogenic factors released by the skin flap ischemia and enhanced tissue regeneration at the lesion site. Consistent with these results, the transplantation of PBM-spheroid hASCs significantly improved functional recovery compared with PBS, monolayer-cultured hASCs, and not-PBM-spheroid treatment. These findings suggest that transplantation of PBM-spheroid hASCs may be an effective stem cell therapy for the treatment of skin flap ischemia.








Similar content being viewed by others
References
Gao W, Ma S, Cui L (2011) Adipose-derived stem cells accelerate neovascularization in ischaemic diabetic skin flap via expression of hypoxia-inducible factor-1α. J Cell Mol Med 12:2575–2585
Tepper OM (2005) Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105:1068–1077
Kim EK, Lee TJ, Hong JP (2011) The effect of human adipose-derived stem cells on healing of ischemic wounds in a diabetic nude mouse model. Plast Reconstr Surg 128:387–394
Shen T, Zhou X, Hong CY (2013) Accelerated healing of diabetic wound using artificial dermis constructed with adipose stem cells and poly(l-glutamic acid)/chitosan scaffold. Chin Med J 126:1498–1503
Park IS, Kim SH (2013) A novel three-dimensional adipose-derived stem cell cluster for vascular regeneration in ischemic tissue. Cytotherapy 13:00681–00686
Korff T (1998) Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 143:1341–1352
Park IS, Jung Y, Rhie JW, Kim SH (2013) Endothelial differentiation and vasculogenesis induced by three-dimensional adipose-derived stem cells. Anat Rec (Hoboken) 296:168–177
Bhang SH, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS (2011) Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32:2734–2747
Park IS, Jung Y, Rhie JW, Kim SH (2013) Endothelial differentiation and vasculogenesis induced by three-dimensional adipose-derived stem cells. Anat Rec (Hoboken) 1:168–177
Valcarcel M, Jaureguibeitia A, Lopategi A, Martinez I, Mendoza L (2008) Three-dimensional growth as multicellular spheroid activates the proangiogenic phenotype of colorectal carcinoma cells via LFA-1-dependent VEGF: implications on hepatic micrometastasis. J Transl Med 6:57
Choi K, Kim H, Lee S, Bae S, Kweon OK, Kim WH (2013) Low-level laser therapy promotes the osteogenic potential of adipose-derived mesenchymal stem cells seeded on an acellular dermal matrix. J Biomed Mater Res B Appl Biomater 101:919–928
Wu Y, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2659
Peplow PV, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29:285–304
Mvula B, Moore T, Abrahamse H (2008) The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med Sci 23:277–282
Hou JF, Yuan X, Li J, Wei YJ, Hu SS (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med 40:726–733
Hu WP, Yu CL, Lan CC, Chen GS, Yu HS (2007) Helium-neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol 127:2048–2057
von Sengbusch A, Fisch KM, Enns A, Nicolson GL, Haier J (2005) Focal adhesion kinase regulates metastatic adhesion of carcinoma cells within liver sinusoids. Am J Pathol 166:585–596
Heydarkhan-Hagvall S, Yang JQ, Heydarkhan S, Xu Y, Zuk PA (2008) Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells Tissues Organs 187:263–274
Lee EJ, Jeon HJ, Kim HS, Chang MS (2013) Potentiated therapeutic angiogenesis by primed human mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen Med 8:283–293
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
Research funder: Research Support Foundation of Ministry of Science, ICT, and Future Planning grant, Basic Science Research Program; Grant number: 2012K1A4A3053142, NRF-2014R1A1A1038199, 2015R1D1A1A02062326, HI14C2161.
The authors declare that they have no conflict of interest with the Research Foundation.
Informed consent
The experiments were done with mice.
Rights and permissions
About this article
Cite this article
Park, IS., Chung, PS., Ahn, J.C. et al. Human adipose-derived stem cell spheroid treated with photobiomodulation irradiation accelerates tissue regeneration in mouse model of skin flap ischemia. Lasers Med Sci 32, 1737–1746 (2017). https://doi.org/10.1007/s10103-017-2239-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2239-1