Abstract
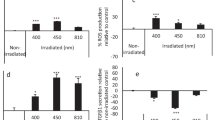
Porphyromonas gingivalis causes chronic inflammatory diseases (periodontal diseases) that destroy the periodontal ligament and alveolar bone. Antimicrobial peptides are crucial components of the host defense response required to maintain cellular homeostasis during microbial invasion. Because light-emitting diode (LED) irradiation influences the host defense response against bacterial infections, we investigated its effect on immortalized gingival fibroblasts (IGFs) infected with P. gingivalis. IGFs were incubated with P. gingivalis following LED irradiation at 425, 525, and 625 nm. The dark 1 group comprised noninfected, nonirradiated IGFs, and the dark 2 group comprised nonirradiated IGFs infected with P. gingivalis. These groups served as controls. Infected cells and controls were assayed for reactive oxygen species (ROS) and were subjected to RT-PCR and Western blotting analyses to determine the levels of expression of antimicrobial peptides. LED irradiation enhanced the bactericidal effects of the antimicrobial peptide LL-37 in cells infected with P. gingivalis. Irradiation at 625 nm decreased inflammatory responses involving the release of prostaglandin E2 induced by ROS in P. gingivalis-infected IGFs. LED irradiation at 625 nm induces an anti-inflammatory response that elicits the production of antimicrobial peptides, providing an efficacious method of treatment for periodontal diseases.





Similar content being viewed by others
References
Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS (1988) Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science 239(4835):55–57
Socransky SS, Haffajee AD (1992) The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol 63(4 Suppl):322–331
Choi JI, Nakagawa T, Yamada S, Takazoe I, Okuda K (1990) Clinical, microbiological and immunological studies on recurrent periodontal disease. J Clin Periodontol 17(7 Pt 1):426–434
Wang M, Hajishengallis G (2008) Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol 10(10):2029–2042. doi:10.1111/j.1462-5822.2008.01185.x
Liu YC, Lerner UH, Teng YT (2010) Teng YT (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontology 2000 52(1):163–206. doi:10.1111/j.1600-0757.2009.00321.x
Zhou Q, Desta T, Fenton M, Graves DT, Amar S (2005) Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun 73(2):935–943. doi:10.1128/IAI. 73.2.935-943.2005
Noguchi K, Yanai M, Shitashige M, Nishihara T, Ishikawa I (2000) Cyclooxygenase-2-dependent prostaglandin production by peripheral blood monocytes stimulated with lipopolysaccharides isolated from periodontopathogenic bacteria. J Periodontol 71(10):1575–1582. doi:10.1902/jop.2000.71.10.1575
Scheres N, Laine ML, de Vries TJ, Everts V, van Winkelhoff AJ (2010) Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis. J Periodontal Res 45(2):262–270. doi:10.1111/j.1600-0765.2009.01229.x
Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD (2008) Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol 153:S241–S246. doi:10.1038/sj.bjp.0707487
Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR (2010) Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med 42(1):38–44. doi:10.1002/lsm.20887
Walker CB (1996) The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000(10):79–88
Hancock REW, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557. doi:10.1038/Nbt1267
Dale BA, Fredericks LP (2005) Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issue Molec Biol 7(2):119–133
Nijnik A, Hancock RE (2009) The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol 16(1):41–47
Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock RE (2005) Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun 73(1):583–591. doi:10.1128/IAI. 73.1.583-591.2005
Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R (2003) An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 111(11):1665–1672. doi:10.1172/JCI17545
Bals R (2000) Epithelial antimicrobial peptides in host defense against infection. Respir Res 1(3):141–150. doi:10.1186/rr25
Barber GN (2001) Host defense, viruses and apoptosis. Cell Death Differ 8(2):113–126. doi:10.1038/sj.cdd.4400823
Rosenfeld Y, Papo N, Shai Y (2006) Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem 281(3):1636–1643. doi:10.1074/jbc.M504327200
Nell MJ, Tjabringa GS, Vonk MJ, Hiemstra PS, Grote JJ (2004) Bacterial products increase expression of the human cathelicidin hCAP-18/LL-37 in cultured human sinus epithelial cells. FEMS Immunol Med Microbiol 42(2):225–231. doi:10.1016/j.femsim.2004.05.013
Kim S, Kim J, Lim W, Jeon S, Kim O, Koh JT, Kim CS, Choi H, Kim O (2013) In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed Laser Surg 31(11):554–562. doi:10.1089/pho.2012.3343
Lockwood DB, Wataha JC, Lewis JB, Tseng WY, Messer RL, Hsu SD (2005) Blue light generates reactive oxygen species (ROS) differentially in tumor vs. normal epithelial cells. Dent Mater 21(7):683–688. doi:10.1016/j.dental.2004.07.022
Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H (2007) The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med 39(7):614–621. doi:10.1002/lsm.20533
Mongardini C, Di Tanna GL, Pilloni A (2014) Light-activated disinfection using a light-emitting diode lamp in the red spectrum: clinical and microbiological short-term findings on periodontitis patients in maintenance. A randomized controlled split-mouth clinical trial. Laser Med Sci 29(1):1–8. doi:10.1007/s10103-012-1225-x
Eick S, Markauskaite G, Nietzsche S, Laugisch O, Salvi GE, Sculean A (2013) Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagn Photodyn 10(2):156–167. doi:10.1016/j.pdpdt.2012.12.001
Kim J, Jung H, Lim W, Kim S, Ko Y, Karna S, Kim O, Choi Y, Choi H, Kim O (2013) Down-regulation of heat-shock protein 27-induced resistance to photodynamic therapy in oral cancer cells. J Oral Pathol Med Off Public Int Assoc Oral Pathol Am Acad Oral Pathol 42(1):9–16. doi:10.1111/j.1600-0714.2012.01155.x
Choi H, Lim W, Kim I, Kim J, Ko Y, Kwon H, Kim S, Kabir KM, Li X, Kim O, Lee Y, Kim S, Kim O (2012) Inflammatory cytokines are suppressed by light-emitting diode irradiation of P. gingivalis LPS-treated human gingival fibroblasts: inflammatory cytokine changes by LED irradiation. Lasers Med Sci 27(2):459–467. doi:10.1007/s10103-011-0971-5
Chui C, Hiratsuka K, Aoki A, Takeuchi Y, Abiko Y, Izumi Y (2012) Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers Surg Med 44(10):856–864. doi:10.1002/lsm.22090
Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M (2001) Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol 22(4):199–204
Ara T, Kurata K, Hirai K, Uchihashi T, Uematsu T, Imamura Y, Furusawa K, Kurihara S, Wang PL (2009) Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J Periodontal Res 44(1):21–27. doi:10.1111/j.1600-0765.2007.01041.x
Vijayalakshmi M, Anand P (2012) A short review: a perspective on the role of prostaglandins (PGE2) on periodontium in health and disease. J Basic Med Allied Sci:1
Noguchi K, Shitashige M, Yanai M, Morita I, Nishihara T, Murota S, Ishikawa I (1996) Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation 20(5):555–568
Ryan LK, Dai JH, Yin ZW, Megjugorac N, Uhlhorn V, Yim S, Schwartz KD, Abrahams JM, Diamond G, Fitzgerald-Bocarsly P (2011) Modulation of human beta-defensin-1 (hBD-1) in plasmacytoid dendritic cells (PDC), monocytes, and epithelial cells by influenza virus, Herpes simplex virus, and Sendai virus and its possible role in innate immunity. J Leukoc Biol 90(2):343–356. doi:10.1189/Jlb.0209079
Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286(5439):525–528
Sahasrabudhe KS, Kimball JR, Morton TH, Weinberg A, Dale BA (2000) Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J Dent Res 79(9):1669–1674
Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale DA (2000) Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun 68(5):2907–2915. doi:10.1128/Iai. 68.5.2907-2915.2000
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298(5595):1025–1029. doi:10.1126/science.1075565
Bals R, Wang XR, Zasloff M, Wilson JM (1998) The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 95(16):9541–9546. doi:10.1073/pnas.95.16.9541
Nizet V, Gallo RL (2003) Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis 35(9):670–676. doi:10.1080/00365540310015629
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government MSIP (2011–0030121) and MEST (NRF-2013R1A1A2061056)
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
JiSun Kim and SangWoo Kim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, J., Kim, S., Lim, W. et al. Effects of the antimicrobial peptide cathelicidin (LL-37) on immortalized gingival fibroblasts infected with Porphyromonas gingivalis and irradiated with 625-nm LED light. Lasers Med Sci 30, 2049–2057 (2015). https://doi.org/10.1007/s10103-014-1698-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1698-x