Abstract
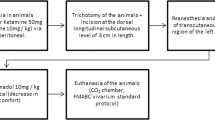
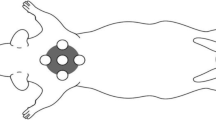
This study aims to investigate the effect of different energy densities provided by low-level laser therapy (LLLT) on the morphology of scar tissue and the oxidative response in the healing of secondary intention skin wounds in rats. Twenty-four male adult Wistar rats were used. Skin wounds were made on the backs of the animals, which were randomized into three groups of eight animals each as follows, 0.9% saline (control); laser GaAsAl 30 J/cm2 (L30); laser GaAsAl 90 J/cm2 (L90). The experiment lasted 21 days. Every 7 days, the wound contraction index (WCI) was calculated and tissue from different wounds was removed to assess the proportion of cells and blood vessels, collagen maturation index (CMI), thiobarbituric acid reactive substance (TBARS) levels and catalase activity (CAT). On the 7th and 14th days, the WCI and the proportion of cells were significantly higher in groups L30 and L90 compared to the control (p < 0.05). At all the time points analyzed, there was a greater proportion of blood vessels and a higher CMI in group L90 compared to the other groups (p < 0.05). On the 7th and 14th days, lower TBARS levels and increased CAT activity were found in the L90 group compared to the control (p < 0.05). On the 7th day, a moderately negative correlation was found between TBARS levels and WCI, CMI and CAT in all the groups. LLLT may modulate the oxidative status of wounded tissue, constituting a possible mechanism through which the LLLT exerts its effects in the initial phases of tissue repair.



Similar content being viewed by others
References
Woodruff LD, Bounkeo J, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS (2004) The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22:241–247
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Photomed Laser Surg 23:289–294
Silveira PCL, Silva LA, Tuon T, Freitas TP, Streck EL, Pinho RA (2009) Effects of low-level laser therapy on epidermal oxidative response induced by wound healing. Braz J Phys Ther 13:281–287
Xavier M, David DR, Souza RA et al (2010) Anti-inflammatory effects of low-level light emitting diode therapy on achilles tendinitis in rats. Lasers Surg Med 42:553–558
Liu H, Dang Y, Wang Z, Chai X, Ren Q (2008) Laser induced collagen remodeling: a comparative study in vivo on mouse model. Lasers Surg Med 40:13–19
Gonçalves RV, Mezêncio JMS, Benevides GP, Matta SLP, Neves CA, Sarandy MM, Vilela EF (2010) Effect of gallium–arsenide laser, gallium–aluminum–arsenide laser and healing ointment on cutaneous wound healing in Wistar rats. Braz J Med Biol Res 43:350–355
Gonçalves RV, Novaes RD, Matta SLP, Benevides GP, Faria FR, Pinto MVM (2010) Comparative study of the effects of gallium–aluminum–arsenide laser photobiomodulation and healing oil on skin wounds in Wistar rats: a histomorphometric study. Photomed Laser Surg 28:597–602
Medrado AP, Pugliese LS, Reis SRA, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Stadler I, Lanzafame RJ, Evans R, Narayan V, Dailey B, Buehner N, Naim JO (2001) 830-nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg Med 28:220–226
Meirelles GCS, Santos NJ, Chagas PO, Moura AP, Pinheiro ALB (2008) A comparative study of the effects of laser photobiomodulation on the healing of third-degree burns: a histological study in rats. Photomed Laser Surg 26:159–166
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, González-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Silveira PC, Streck EL, Pinho RA (2007) Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol 86:279–282
Servetto N, Cremonezzi D, Simes JC, Moya M, Soriano F, Palma JA, Campana VR (2010) Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers Surg Med 42:577–583
Khodr B, Khalil Z (2001) Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radic Biol Med 30:1–8
Eells JT, Wong-Riley MT, VerHoeve J et al (2004) Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 4:559–567
Kim YG, Pal SC, Lee SR (2000) Hairless mouse epidermal antioxidants and lipid peroxidation assessed by He–Ne laser. Lasers Surg Med 27:420–426
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol 49:1–17
Poli G, Parola M (1997) Oxidative damage and fibrogenesis. Free Radic Biol Med 22:287–305
Agren MS, Mertz PM, Franzén LA (1997) Comparative study of three occlusive dressing in the treatment of full-thickness wounds in pigs. J Am Acad Dermatol 36:53–58
Junqueira LCU, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. Ann Acad Braz Sci 75:469–486
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Gutteridge JMC, Halliwel B (1990) The measurement and mechanism of lipid peroxidation in physiological systems. Trends Biochem 15:129–135
Bradford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Corazza AV, Jorge J, Kurachi C, Bagnato VS (2007) Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 25:102–106
Karu T (1987) Photobiological fundamentals of low-power laser therapy. IEEE J Quant Electron 23:1703–1717
Karu TI (2000) Mechanisms of low-power laser light action on cellular level. In: Simunovic Z (ed) Lasers in medicine and dentistry, 1st edn. Vitgraph, Rijeka, pp 97–125
Webb C, Dyson M (2003) The effect of 880-nm low level laser energy on human fibroblast cell numbers: a possible role in hypertrophic wound healing. J Photochem Photobiol 70:39–44
Steinstraesser L, Wehner M, Trust G et al (2010) Laser-mediated fixation of collagen-based scaffolds to dermal wounds. Lasers Surg Med 42:141–149
Nascimento PM, Pinheiro ALB, Salgado MAC, Ramalho LMP (2004) A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats. Photomed Laser Surg 22:513–518
Mendez TMTV, Pinheiro ALB, Pacheco MTT, Nascimento PM, Ramalho LMP (2004) Dose and wavelength of laser light have influence on the repair of cutaneous wounds. J Clin Laser Med Surg 22:19–25
Saperia D, Gassberg E, Lyons RF, Abergel RP, Baneux P, Castel JC, Dwyer RM, Uitto J (1986) Demonstration of elevated type I and type III procollagen mRNA levels in cutaneous wounds treated with helium–neon laser. Biochem Biophys Res Commun 138:1123–1128
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med 22:281–287
Polosukhin VV (1999) Ultrastructural study of the destructive and repair processes in pulmonary inflammation and following endobronchial laser therapy. Virchows Arch 435:13–19
Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V (2005) The absence of reactive oxygen species production protects mice against bleomycine-induced pulmonary fibrosis. Respir Res 6:1–12
Park SK, Kim J, Seomun Y et al (2001) Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem Biophys Res Commun 284:966–971
Iglesias-De La Cruz MC, Ruiz-Torres MP, De Lucio-Cazana FJ, Rodriguez-Puyol M, Rodriguez-Puyol D (2000) Phenotypic modifications of human mesangial cells by extracellular matrix: the importance of matrix in the contractile response to reactive oxygen species. Exp Nephrol 8:97–103
Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA (2000) Reactive oxygen species, signaling and cell injury. Free Radic Biol Med 10:1456–1462
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves, R.V., Novaes, R.D., do Carmo Cupertino, M. et al. Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med Sci 28, 383–390 (2013). https://doi.org/10.1007/s10103-012-1066-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1066-7