Abstract
Fossil fuels are the primary energy source of almost all societies and economies, but it is finite and scarce. The use of non-renewable fossil fuels threatens earth’s environment. At the same time, waste from agricultural and industrial activities is increasing. Most of this waste is discarded or poorly managed, causing many other environmental issues. Converting waste to energy is a promising route to address these challenges. We investigated the hydrothermal liquefaction (HTL) of high moisture content, tobacco-processing waste in a multiple batch thermal reactor to produce biocrude oil. The effects of operating conditions were studied and optimized for maximum liquid biocrude oil yield. HTL operating conditions considered were temperatures from 280 to 340 °C and residence times from 15 to 45 min for a fixed ratio of biomass to deionized water of 1:3. The reaction temperature was found to affect the yields and distribution of products significantly. The maximum yield of the liquid biocrude oil obtained was more than 52% w/w at 310 °C and 15 min. Under these conditions, almost 90% of the energy was recovered in biocrude oil and solid products. The liquid fraction was mainly composed of phenols, ketones, and nitrogenous compounds. This study provides a potential framework for eco-technologies for biomass waste-to-energy conversion with respect to converting tobacco processing residues to liquid biofuels and biochemicals.
Graphic abstract

Similar content being viewed by others
Introduction
Fossil fuels are used to produce energy worldwide, but they come with major problems such as global warming (Zhang and Liu 2021). To reduce the use of fossil fuels, biomass-based fuels offer a great potential alternative since biomass is one of the most promising renewable and sustainable energy resources (Houshfar et al. 2014; Singh and Dhepe 2018; Wang et al. 2021) While biomass waste has increased markedly due to the intensification of agricultural and industrial activities to serve a growing population, its disposal, utilization, and management practices are not efficiently employed (Tripathi et al. 2019). Biomass waste is usually discarded to degrade or to be burned in open areas, resulting in haze that harms the local environment (Madhu et al. 2018). Biomass waste-to-energy conversion is a promising solution for the above-mentioned challenges.
In the past decades, biomass conversion processes have been extensively studied for converting biomass into liquid biofuel via gasification (Fan et al. 2020), pyrolysis (Onsree et al.2018a, 2018b, 2019; Onsree and Tippayawong 2020a), and hydrothermal processing (Kantakanit et al. 2018). Among these processes, only hydrothermal liquefaction (HTL) can be efficiently applied to convert high moisture content or wet biomass materials generally having a moisture content of more than 50% w/w, such as fresh biowaste, food waste, algal biomass, and wastewater sludge to biocrude oil without a drying requirement. HTL is a thermochemical process in the presence of a solvent at moderate temperatures (200–374 °C) and high pressures, between 5 and 20 MPa (Ponnusamy et al. 2020). The process undergoes reactions similar to the formation of underground fossil fuels, but with far shorter reaction times, requiring merely a few minutes or hours, compared to the millions of years of the fossilization process (Dimitriadis and Bezergianni 2017; Gollakota et al. 2018). By using HTL, over two-thirds of the carbon content in biomass could be recovered as either biochar or biocrude oil, with expected yields of more than 40% w/w and heating values ranging from 30 to 40 MJ/kg (Tekin et al. 2014; Yang et al. 2019). One of the challenges in utilizing the HTL process is that a high-pressure reactor is required, making the process complicated and costly (Zhang et al. 2010).
Rather than simply maintaining a high pressure, materials used to build the reactor have to withstand extremely corrosive environments during the hydrothermal reactions (Zhang et al. 2010). In general, there are two types of HTL reactors: batch and continuous (Déniel et al. 2016a; Peterson et al. 2008). Construction of a continuous system presents further technical challenges, such as feedstock loading, in which feeding the slurry into the system at a certain concentration is a complex task (Déniel et al. 2016a). In other words, the system requires the development of methods for feeding raw biomass to the process inlet as well as for pumping highly viscous biocrude oil at the outlet. Elliott et al. (2015) noted that continuous HTL reactor technology has significant commercialization potential, but several challenges remain that need to be addressed before this can occur. To gain insight into the mechanism of HTL, experiments in a batch reactor are still necessary before progressing to commercial scales. Small batch reactors (10–1000 mL) are mostly used in laboratories, as operating conditions and feedstock loadings are easily controlled. For example, Jensen et al (2017) used a 10 mL micro-batch reactor to probe the HTL of lignocellulosic biomass. They obtained biocrude oil yields as high as 45% w/w. Xu and Lad (2008) employed a 14 mL bomb reactor to study the HTL of Jack pine sawdust and reported that the maximum liquid biocrude oil obtained without using catalysts was over 50% w/w at 300 °C and 30 min. Jindal and Jha (2016) used a 600 mL batch reactor to investigate the HTL of waste furniture sawdust and found that the biocrude oils had improved HHVs up to between 17 and 30 MJ/kg. Arun et al. (2021), who investigated the HTL of Prosopis juliflora biomass, found that the optimum biocrude oil yield was 22.3% w/w at 300 °C, 60 min, and a 1:20 biomass to solvent ratio. Wang et al (2019) carried out HTL of waste Tetra Pak in sub- and supercritical water in a micro-batch reactor (100 mm long and 7.5 mm in diameter). They found that the maximum yield of biocrude oil was about 36% w/w at 360 °C, 22 MPa, 30 min, with a feed concentration of 20% w/w, and their biocrude oil mainly contained ketones, phenolics, esters, and alcohols.
Tobacco is a major economic crop in many countries. In Thailand, its processing is mainly managed by the Thai Tobacco Authority with reported revenue in the range of US$1.65 billion annually. Prior to the current covid-19 pandemic period, its growth was about 2.4–4.2% a year (Thai Tobacco 2021). There are large amounts of wastes and residues generated from tobacco processing. Since they are usually considered worthless, the waste is either heaped on farmlands or burnt on-site for disposal. So far, only a few papers on converting tobacco residues to biocrude oil have been reported (Chumsawat and Tippayawong 2020; Khuenkaeo et al. 2020). Pyrolysis was used as the main conversion process in all these papers. Their results indicated that using pyrolysis, a high reaction temperature of about 600 °C was necessary to maximize the biocrude oil yield, compared to the HTL reaction which only needs temperatures between 280 and 340 °C. Moreover, published reports on HTL of agricultural residues, especially tobacco processing waste, remain scarce.
Therefore, the specific objective of this work was to investigate the thermal conversion of tobacco processing waste to biocrude oil by HTL in a multiple batch reactor. The operating conditions considered were reaction temperatures of 280, 310, and 340 °C, and residence times of 15, 30, and 45 min at a fixed biomass-to-solvent ratio of 1:3. The distribution and yields of liquid-, solid-, and gas-phase products were examined across those operating conditions. For each condition, at least three repeats were performed. The main components of the biocrude oil were also analyzed.
Materials and methods
Biomass sample and chemicals
Tobacco processing residues, provided by the Tobacco Authority of Thailand‘s Denchai redrying plant in Phrae, Thailand, were used as the biomass sample. Prior to the tests, all dirt and impurities were manually removed from the waste sample. It was visually inspected to ensure no contamination occurred. It was then ground to approximately 3–5 mm in diameter. The samples were dried in an oven at 105 °C for 24 h and cooled to room temperature in a controlled humidity chamber. The dried samples were stored in Ziplock bags prior to being used in any experiments. Deionized (DI) water was used as the solvent for the HTL reactions. Dichloromethane (DCM), acetone, and methanol with over 99% purity, purchased from RCI Labscan, were used in product extractions and chemical composition analysis processes.
HTL reactor setup
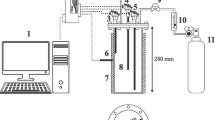
HTL reactions occur at high pressure (5–20 MPa) and moderate temperature (200–374 °C) (Ponnusamy et al. 2020). During the reactions, a mixture of solvent and biomass may produce corrosive substances inside the reactor (Zhang et al. 2010). In the present work, four custom-built identical batch container vessels were used. Each was designed with an internal volume of 200 mL for reaction pressures and temperatures up to 22 MPa and 400 °C, respectively. Each container vessel was made of high-quality stainless steel (SS-304), in which the container body was tightly sealed with a steel flange and six bolts, as shown in Fig. 1.
The container vessels were heated by an external high-temperature muffle furnace (Dongguan LIYI Environmental Technology Co., Ltd., China), generating a maximum temperature of 900 °C. For safety, a rupture disk was installed to prevent equipment failure in the case of excessive internal pressure (25 MPa). For each container, the biomass sample was mixed with DI water at a fixed mass ratio of 1:3. All reaction vessels were tested at maximum temperature and pressure conditions: the batch reactor was filled with 20 ± 1 g of tobacco residues and 60 ± 2 g of DI water to simulate HTL reactions.
Monitoring the pressures and temperatures inside the batch containers during the reactions was complicated. In this work, a dummy batch container was deployed and installed inside the oven chamber. It was mounted and connected to a Huadong pressure gauge (< 1% full scale) and a digital Sensoheat K-type thermocouple (± 0.75% of reading). During each test, the dummy batch reactor was filled with a mixture of biomass and DI water similar to the tested samples. The pressure and temperature inside the dummy batch reactor were regularly measured and recorded as a function of time and considered representative for all batch containers. The uncertainty of the measurement and the results from other batches compared to the dummy were within 5%. The slurry mixture in the batch reactors was expected to be in a near-critical fluid state. When the temperature of the mixture was increased, the pressure increased monotonically, according to saturation. Therefore, in this study, only the temperature was controlled and varied, and the reaction time was considered to begin after the temperature of the dummy container reached the set point.
HTL procedure and product extraction
In our HTL reactor, the biomass sample and DI water were mixed and stirred vigorously to make sure that the mixture was homogeneous. Then, the container vessels were heated to a temperature (280, 310, and 340 °C) in an oven for a specific reaction time of 15, 30, and 45 min. Next, the container vessels were rapidly cooled to room temperature in a cold-water bath, immediately halting the reactions, similar to previous work (Aierzhati et al. 2019).
Figure 2 shows the product extraction. After the HTL reactions ended, the gas-phase product in each batch container was vented to the atmosphere at room conditions, and the remaining reaction mixture was carefully collected. In line with the previous reports (Caprariis et al. 2017; Jiang and Savage 2017; Valdez et al. 2012; Xiu et al. 2010; Zhou et al. 2010), the solid and liquid products were separated using filter paper under vacuum. The filtrate was subsequently extracted with an equal quantity of DCM, and the resulting suspension consisted of two layers: (i) DCM-soluble and (ii) water-soluble fractions (Valdez et al. 2012). The DCM solution was evaporated at 38 °C (Jiang and Savage 2017), while the water-soluble fraction was evaporated at 65 °C for 12 h to completely remove the water (Xiu et al. 2010). The organic fractions from the filtrate which remained were labeled as light biocrude oil (LBO) and water-soluble (WS) fractions, respectively. The solid product remaining on the filter paper was washed with acetone until the solvent became colorless, and then it was dried at 105 °C for 24 h to obtain the solid product (Zhou et al. 2010). After removing the acetone from the washing liquid in an evaporator at 60 °C, this fraction was quantified and labeled as the heavy biocrude oil (HBO) (Caprariis et al. 2017). The total liquid biocrude oil yield was a sum of LBO, HBO, and WS. With the total initial biomass loading mass (\(M\)), the yield of each product can be calculated (on a dry basis) from Eqs. 1 and 2:
where \(y\) and \(m\) are the yield and mass of each product, respectively, the subscript \(i\) refers to the liquid and solid products.
Product analyses
The moisture content, ultimate composition, and higher heating value of the tobacco residues and solid products were analyzed according to the ASTM D3173, D5373, and D5865 standards. The ultimate analysis of the organic liquid products (HBO, LBO, and WS) was performed using a ThermoScientific CHNS/O analyzer (Flash 2000, Thermo Scientific, Italy), while the chemical composition was analyzed using a gas chromatograph-mass spectrometer (GC: Agilent 7890B and MS: Agilent MSD 5977B, USA) with a DB5-MS column (30 m/0.25 mm/0.50 μm) and an automated liquid sampler (Agilent 7693A). The HBO and LBO fractions were diluted with acetone, and the WS product was diluted with methanol. Approximately 1 μL of the sample mixture was injected into the column with a split ratio of 10:1. Helium was used as the carrier gas at a flow rate of 10 mL/min. The oven temperature was initially set to 60 °C with a hold time of 5 min; then, it was increased at a constant rate of 5 °C/min until it reached 280 °C, where it was held for 5 min. The injector and detector temperatures were set at 250 and 280 °C, respectively. The compounds were identified by comparison with the NIST mass spectral database.
The higher heating value (HHV) of the liquid products was approximated by Eq. 3 (Rajagopal et al. 2021):
where \(C\), \(H\), and \(O\) are the carbon, hydrogen, and oxygen contents of each liquid product (in w/w). The energy recovery (\(ER\)) of the products was calculated from Eq. 4 (Mishra and Mohanty 2020):
where \({HHV}_{tobacco}\) is the higher heating value of the tobacco waste.
Results and discussion
Temperature and pressure evolution in the container vessel
Figure 3 shows the temperature and pressure evolution profile obtained from a representative HTL of tobacco residues and saturated water in a batch container. The x-axis represents the temperatures of the ingredients inside the container. The y-axis is the pressure inside the container at any temperature. As expected, the mixture of tobacco residue and DI water solvent experienced a near saturated state. But the thermodynamic state of the slurry mixture appeared to have slightly higher pressures for temperatures above 200 C°. For example, at 230 °C, the mixture was at 3.0 MPa, whereas pure water saturation at this temperature is about 2.7 MPa. The difference was clearer for temperatures above 260 °C, and an average difference of approximately 1.3 MPa was maintained. In the mixtures, this may have been due to the initiation of the thermal decomposition of the lignocellulosic biomass at temperatures of about 200 °C, which generated many different substances in the container (Tippayawong et al. 2019). Higher temperatures increased the thermal decomposition of biomass (Onsree and Tippayawong 2020b), leading to a more complicated mixture in the batch container. The resulting pressure subsequently rose according to the saturation of some of those new substances (Déniel et al. 2016b).
Distribution and yields of HTL products
Figure 4 shows the major products in this study were liquid-, solid-, and gas-phase, which were calculated on a dry basis. Both temperature and residence time were found to affect the distribution and yields of HTL products. Increasing the temperature from 280 to 310 °C increased the liquid product yields but lowered the solid and gas product yields. When the reaction temperature increased to 340 °C, liquid biocrude oil yields decreased markedly, leading to a considerable increase in gas formation, while the solid yields were only slightly changed. The negative effect of temperature on HTL liquid products was consistent with previous publications on different biomass resources, such as (Zhu et al. 2015; Yin et al. 2010; Sugano et al. 2008), which established that the reaction temperature range of 300–315 °C was suitable for efficient production of biocrude oil. The effect of temperature on the variability of liquid product yields was due to the complex reaction mechanisms of biomass composition in terms of cellulose, hemicellulose, and lignin (Peterson et al. 2008; Zhang 2010). The decomposition of these three components in DI water at high temperatures was reported to give different products (Toor et al. 2011). Hemicellulose and cellulose decompose at about 220 and 280 °C, respectively, while lignin decomposes over a wide range, 200–500 °C. Hemicellulose and cellulose are beneficial for liquid products, while a high lignin content leads to a high amount of solid products (Zhong and Wei 2004). The decomposition mechanism during the HTL reactions consisted mainly of the following three steps: depolymerization, decomposition, and recombination (Toor et al. 2011). At the initial stage, the biomass was decomposed and depolymerized to form small compounds. These compounds may rearrange through condensation, cyclization, and polymerization to form new compounds (Sun et al. 2010). In other words, an increase in temperature triggered the liquid yields. After reaching the maximum yield of the liquid product, a further increase in temperature inhibited the HTL of the biomass, promoted the decomposition of all the compounds into gaseous products and possibly allowed the repolymerization or condensation of the intermediates into chars (Akhtar and Amin 2011). The reaction temperature to maximize the liquid product yield has previously been found to be slightly above 300 °C (Yin et al. 2010). In this work, the maximum yield of the liquid products was obtained (52.1% w/w) at 310 °C, the optimal conversion temperature for biocrude oil production from HTL of tobacco processing residues.
The residence time was also observed to have a considerable effect on the distribution and yields of HTL products. In the present work, it was defined as the time which the biomass spent in the reactor after reaching the set point temperature. The times for heating up and cooling down the reactor were not included. From Fig. 4, at a reaction temperature of 280 °C, the maximum yield of liquid products (51.7% w/w) was obtained at a residence time of 30 min. However, when the temperature was raised to 310 °C, the maximum yield increased to 52.1% w/w at a residence time of only 15 min. This appeared to be the optimal condition for producing the highest yield of HTL liquid products from tobacco residues based on a combination of reaction temperature and time. At 340 °C, the liquid product yields decreased to 37% w/w.
In contrast, the gas-phase product yields increased slightly with an extended period of residence time. The highest gas product yield was found at 45 min and 340 °C. The solid yield was not significantly affected by the residence time. This indicated that the liquid products reached their maximum before decreasing at longer residence times, whereas the gas yields continuously increased until the saturation point was reached, similar to previous work (Jindal and Jha 2016). There appeared to be an interrelationship between the temperature and residence time needed to maximize the yield of the liquid products. A higher temperature required a lower residence time. However, too short a duration could lead to incomplete degradation and polymerization reactions, while too long a duration caused polymerization of intermediates (Cao et al. 2017). Under extended residence times, the lower yield of the liquid products could be due to the cracking reactions of liquid products to gas-phase products and the formation of char products by condensation (Karagöz et al. 2005). When considering the residence time, the yields of the liquid products in our study were comparable to those reported by Yang et al (2016) and Jena et al (2011).
In order to gain insight into the combined effects of temperature and residence time, Fig. 5 shows the proportions of HBO, LBO, and WS in the liquid products obtained at varying conditions of temperature and residence time. With an increase in temperature from 280 to 310 °C, the HBO fraction increased substantially from 18.9 to 29.6% w/w, whereas the LBO and WS fractions had slight changes with average values of approximately 9.7 and 12.8% w/w, respectively. Compared to a previous study (Rajagopal et al. 2021), the trend of increasing biocrude oil production was the same, except for the WS fraction, which was reported to decrease at a higher temperature. By increasing the residence time from 15 to 45 min, both biocrude oil fractions increased slightly. The WS fraction decreased to 8.7% w/w. When only liquid products were considered, tobacco processing residues hydrothermally decomposed to generate LBO and WS fractions at a low temperature and generate more of the HBO fraction at higher temperatures. An extended residence time did not increase either of the biocrude oil yields, but caused the depolymerization of the light WS components, lowering the liquid yields (Aierzhati et al. 2019; Mishra and Mohanty 2020).
Characterization of HTL products
Table 1 shows the ultimate and HHV analyses of the tobacco waste and HTL products and energy recovery of each product. Compared to the raw biomass material, both of the biocrude oils were rich in C (> 65% w/w) and H (> 7% w/w), as well as low in O (< 26% w/w). The solid product had a C content of up to 70% w/w and an O content of about 24% w/w. Atomic O/C and H/C ratios of these HTL products were observed to be 0.8–1.4 and 0.2–0.3, respectively, which were significantly lower than those of tobacco processing residues. The decreased O/C and H/C ratios of the HTL products were due to deoxygenation and decarboxylation of the biomass feedstock during the liquefaction process (Chen et al. 2019). This also resulted in an increased HHV of the HTL products, which was over 28 MJ/kg for the biocrude oils and about 26 MJ/kg for the solid products. The superior HHV indicated an energy recovery of almost 90% from the raw material. In this case, the HBO and LBO products accounted for more than 65% of the energy recovered. This intensification of energy density in the biofuels is an essential factor for sustainable energy conversion processes. Meanwhile, the WS fraction was found to have a high O content, 80% w/w, with only 11% w/w C and an HHV of only 1.4 MJ/kg HHV. This result was similar to that reported in previous work (Aierzhati et al. 2019).
The HBO, LBO, and WS products were analyzed by GC–MS. The peak values from this analysis do not represent the actual concentrations of compounds, they indicate the product distributions associated with the solvent (Khuenkaeo et al. 2020). Figure 6 depicts the main composition of each liquid product at the optimum reaction condition (310 °C and 15 min). Other compounds with low boiling points may have been present but were covered by solvent peaks or may have been lost during acetone evaporation. Based on the relative percentage of the peak areas, the HTL products mainly contain phenols, ketones, and nitrogenous compounds. The HBO product had the highest proportion of phenols at about 30%, while the LBO fraction showed the highest proportion in ketones at over 32%. Nitrogenous compounds were found to be the most abundant in the WS product at over 35%. The compounds of the liquid products found here were in agreement with those from previous research (Jindal and Jha 2016; Kim and Um 2020), where phenols, carboxylic acids, aromatic hydrocarbons, ketones, aldehydes, and nitrogenous ring structures were reported to be the main components of liquid products from HTL of lignocellulosic biomass. However, the produced HTL biocrude oil in the present work differed from the biocrude oils obtained from food waste and algae with high protein and carbohydrate contents (Chen et al. 2019; Aierzhati et al. 2019; Toor et al. 2013), in which their components consisted mainly of aromatics, phenolic derivatives, carboxylic acids, esters, and nitrogenous ring structures. It was likely that the compounds of HTL biocrude oil products were also influenced by the raw material compositions, apart from the HTL conditions. Attempts have been made to propose possible formation pathways of biocrude oils from HTL of lignocellulosic biomass (Yang et al. 2015). During HTL, polysaccharides, basic components of lignocellulose, are decomposed to phenols and ketones (Kumar et al. 2018), as shown in Fig. 7a. Proteins which could be present in the plant cells can be decomposed into low molecular weight compounds such as pyrroles, pyrazines, and amines. Shown in Fig. 7b for N-containing compounds, proteins are decomposed to amino acids, and then the amino acids further decompose either to carbonic acids and amines by decarboxylation or to ammonia and organic acids by deamination reaction (Peterson et al. 2008). Phenolic compounds are likely derived from the degradation of lignin, while the ketones are probably formed from further reactions of the sugars generated by the degradation of hemicellulose and cellulose (Barbier et al. 2012). Large amount of nitrogenous compounds observed in the WS product may be due to the fact that the tobacco processing residues are initially rich in N content (Khuenkaeo et al. 2020), compared to other types of lignocellulosic biomass (Tanyaket et al. 2020).
Possible formation pathways of biocrude oils from a polysaccharides and b proteins as proposed by Yang et al. (2015). The dotted boxes refer to the compound products in our biocrude oil products
Practical application of HTL as a clean technology
HTL has been shown to be a promising clean technology in converting organic materials into value added oils. The technology can be adopted to support Thailand’s waste management system and its goal to achieve zero waste by 2030, as well as the country’s bio-circular economic policy in reusing, recycling and utilizing waste and converting these resources into value added products. Further research and development in HTL will help propel the future demonstration of this clean technology in managing wet waste. Rather than discarding about 2500 tons of tobacco processing waste annually, the HTL technology may be adopted to convert this waste to biofuels and biochemicals. Apart from tobacco processing waste, the technology can also be applied to other wet organic wastes such as other agricultural residues, food waste, waste from fresh markets, and the organic fraction of municipal solid waste.
Conclusion
Hydrothermal liquefaction (HTL) can be used to convert tobacco processing waste to biomass-based biocrude oil, effectively. By using a batch reactor, the maximum biocrude oil yields over 52% w/w with about 90% of energy recovery at conditions of 310 °C, 15 min and 1:3 biomass to DI water ratio. The main components of the biocrude oils were phenols and ketones. These findings provide a positive outlook for utilizing wet agricultural waste for energy and chemical productions via HTL as a clean technology. Further studies on separation, purification and refining of highly valuable components or upgrading of the biocrude oils to biofuels should be considered and undertaken.
Data availability
Data will be made available upon request.
References
Aierzhati A, Stablein MJ, Wu NE, Kuo CT, Si B, Kang X, Zhang Y (2019) Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour Technol 284:139–147
Akhtar J, Amin NAS (2011) A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 15(3):1615–1624
Arun J, Gopinath KP, Sivaramakrishnan R, Shyam S, Mayuri N, Manasa S, Pugazhendhi A (2021) Hydrothermal liquefaction of Prosopis juliflora biomass for the production of ferulic acid and bio-oil. Bioresour Technol 319:124116. https://doi.org/10.1016/j.biortech.2020.124116
Barbier J, Charon N, Dupassieux N, Loppinet-Serani A, Mahé L, Ponthus J, Courtiade M, Ducrozet A, Quoineaud AA, Cansell F (2012) Hydrothermal conversion of lignin compounds. a detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 46:479–491
Cao L, Zhang C, Chen H, Tsang DCW, Luo G, Zhang S, Chen J (2017) Hydrothermal liquefaction of agricultural and forestry wastes: state-of-the-art review and future prospects. Bioresour Technol 245:1184–1193
Caprariis B, Filippis P, Petrullo A, Scarsella M (2017) Hydrothermal liquefaction of biomass: influence of temperature and biomass composition on the bio-oil production. Fuel 208:618–625
Chen WHH, Lin YYY, Liu HCC, Chen TCC, Hung CHH, Chen CHH, Ong HC (2019) A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl Energy 237:283–291
Chumsawat L, Tippayawong N (2020) Utilizing tobacco residues to generate bio-oil and biochar via ablative pyrolysis. Chem Eng Trans 78:49–54
Déniel M, Haarlemmer G, Roubaud A, Weiss-Hortala E, Fages J (2016a) Energy valorisation of food processing residues and model compounds by hydrothermal liquefaction. Renew Sustain Energy Rev 54:1632–1652
Déniel M, Haarlemmer G, Roubaud A, Weiss-Hortala E, Fages J (2016b) Optimisation of bio-oil production by hydrothermal liquefaction of agro-industrial residues: blackcurrant pomace (Ribes nigrum L.) as an example. Biomass Bioenergy 95:273–285
Dimitriadis A, Bezergianni S (2017) Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: a state of the art review. Renew Sustain Energy Rev 68:113–125
Elliott DC, Biller P, Ross AB, Schmidt AJ, Jones SB (2015) Hydrothermal liquefaction of biomass: developments from batch to continuous process. Bioresour Technol 178:147–156
Fan Y, Tippayawong N, Wei G, Huang Z, Zhao K, Jiang L, Zheng A, Zhao Z, Li H (2020) Minimizing tar formation whilst enhancing syngas production by integrating biomass torrefaction pretreatment with chemical looping gasification. Appl Energy 260:114315. https://doi.org/10.1016/j.apenergy.2019.114315
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392
Houshfar E, Wang L, Vähä-Savo N, Brink A, Løvås T (2014) Characterisation of CO/NO/SO2 emission and ash-forming elements from the combustion and pyrolysis process. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-014-0762-3
Jena U, Das KC, Kastner JR (2011) Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour Technol 102(10):6221–6229
Jensen CU, Rosendahl LA, Olofsson G (2017) Impact of nitrogenous alkaline agent on continuous HTL of lignocellulosic biomass and biocrude upgrading. Fuel Process Technol 159:376–385
Jiang J, Savage PE (2017) Influence of process conditions and interventions on metals content in biocrude from hydrothermal liquefaction of microalgae. Algal Res 26:131–134
Jindal MK, Jha MK (2016) Effect of process parameters on hydrothermal liquefaction of waste furniture sawdust for bio-oil production. RSC Adv 6(48):41772–41780
Kantakanit P, Tippayawong N, Koonaphapdeelert S, Pattiya A (2018) Hydrochar generation from hydrothermal carbonization of organic wastes. IOP Conf Series Earth Environ Sci 159(1):12001
Karagöz S, Bhaskar T, Muto A, Sakata Y, Oshiki T, Kishimoto T (2005) Low-temperature catalytic hydrothermal treatment of wood biomass: Analysis of liquid products. Chem Eng J 108(1–2):127–137
Khuenkaeo N, MacQueen B, Onsree T, Daiya S, Tippayawong N, Lauterbach J (2020) Bio-oils from vacuum ablative pyrolysis of torrefied tobacco residues. RSC Adv 10(58):34986–34995
Kim SJ, Um BH (2020) Effect of thermochemically fractionation before hydrothermal liquefaction of herbaceous biomass on biocrude characteristics. Renew Energy 160:612–622
Kumar M, Olajire OA, Kumar A (2018) A review on the current status of various hydrothermal technologies on biomass feedstock. Renew Sustain Energy Rev 81:1742–1770
Madhu P, Kumar C, Anojkumar L, Waran M (2018) Selection of biomass materials for bio-oil yield: a hybrid multi-criteria decision making approach. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-018-1545-z
Mishra S, Mohanty K (2020) Co-HTL of domestic sewage sludge and wastewater treatment derived microalgal biomass—an integrated biorefinery approach for sustainable biocrude production. Energy Convers Manage 204:112312. https://doi.org/10.1016/j.enconman.2019.112312
Onsree T, Tippayawong N (2020a) Torrefaction of maize residue pellets with dry flue gas. Bioenergy Res 13(1):358–368
Onsree T, Tippayawong N (2020b) Analysis of reaction kinetics for torrefaction of pelletized agricultural biomass with dry flue gas. Energy Rep 6:61–65
Onsree T, Sittisun P, Sasaki R, Tippayawong N (2018) Pyrolysis of corn residues: kinetic analysis using discrete distributed activation energy model. IOP Conf Series Earth Environ Sci. https://doi.org/10.1088/1755-1315/159/1/012036
Onsree T, Tippayawong N, Zheng A, Li H (2018b) Pyrolysis behavior and kinetics of corn residue pellets and eucalyptus wood chips in a macro thermogravimetric analyzer. Case Stud Thermal Eng. https://doi.org/10.1016/j.csite.2018.07.011
Onsree T, Tippayawong N, Williams T, McCullough K, Barrow E, Pogaku R, Lauterbach J (2019) Torrefaction of pelletized corn residues with wet flue gas. Bioresour Technol 285:121330. https://doi.org/10.1016/j.biortech.2019.121330
Peterson AA, Vogel F, Lachance RP, Fröling M, Antal MJ, Tester JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ Sci 1(1):32–65
Ponnusamy VK, Nagappan S, Bhosale RR, Lay CH, Nguyen D, Pugazhendhi A, Chang SW, Kumar G (2020) Review on sustainable production of biochar through hydrothermal liquefaction: physico-chemical properties and applications. Bioresour Technol 310:123414. https://doi.org/10.1016/j.biortech.2020.123414
Rajagopal J, Gopinath KP, Krishnan A, Vikas MN, Arun J (2021) Photocatalytic reforming of aqueous phase obtained from liquefaction of household mixed waste biomass for renewable bio-hydrogen production. Bioresour Technol 321:124529. https://doi.org/10.1016/j.biortech.2020.124529
Singh S, Dhepe P (2018) Effect of structural properties of organosolv lignins isolated from different rice husks on their liquefaction using acidic ionic liquids. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-017-1435-9
Sugano M, Takagi H, Hirano K, Mashimo K (2008) Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J Materials Sci 43(7):2476–2486
Sun P, Heng M, Sun S, Chen J (2010) Direct liquefaction of paulownia in hot compressed water: influence of catalysts. Energy 35(12):5421–5429
Tanyaket T, Onsree T, Tippayawong N, Baratieri M (2020) Effect of oxidative torrefaction on characteristics of treated corncob pellets. J Chinese SOC Mech Eng 41(1):65–73
Tekin K, Karagöz S, Bektaş S (2014) A review of hydrothermal biomass processing. Renew Sustain Energy Rev 40:673–687
Tippayawong N, Onsree T, Williams T, McCullough K, MacQueen B, Lauterbach J (2019) Catalytic torrefaction of pelletized agro-residues with Cu/Al2O3 catalysts. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00535-w
Thai Tobacco (2021) Operation results. Available online at https://thaitobacco.or.th
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 36(5):2328–2342
Toor SS, Reddy H, Deng S, Hoffmann J, Spangsmark D, Madsen LB, Holm-Nielsen JB, Rosendahl LA (2013) Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour Technol 131:413–419
Tripathi N, Hills CD, Singh RS, Atkinson CJ (2019) Biomass waste utilisation in low-carbon products: harnessing a major potential resource. npj Clim Atmos Sci 2(1):1–10
Valdez PJ, Nelson MC, Wang HY, Lin XN, Savage PE (2012) Hydrothermal liquefaction of Nannochloropsis sp.: systematic study of process variables and analysis of the product fractions. Biomass Bioenergy 46:317–331
Wang Y, Zhu Y, Liu Z, Su J, Fang C, Xu D, Song W, Wang S (2019) Influences of operating parameters on liquefaction performances of Tetra Pak in sub-/supercritical water. J Environ Manage 237:545–551
Wang S, Zhao S, Cheng X, Qian L, Barati B, Gong X, Cao B, Yuan C (2021) Study on two-step hydrothermal liquefaction of macroalgae for improving bio-oil. Bioresour Technol 319:124176. https://doi.org/10.1016/j.biortech.2020.124176
Xiu S, Shahbazi A, Shirley V, Cheng D (2010) Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J Anal Appl Pyrol 88(1):73–79
Xu C, Lad N (2008) Production of heavy oils with high caloric values by direct liquefaction of woody biomass in sub/near-critical water. Energy Fuels 22(1):635–642
Yang W, Li X, Li Z, Tong C, Feng L (2015) Understanding low-lipid algae hydrothermal liquefaction characteristics and pathways through hydrothermal liquefaction of algal major components: crude polysaccharides, crude proteins and their binary mixtures. Bioresour Technol 196:99–108
Yang L, Nazari L, Yuan Z, Corscadden K, Xu CC, He QS (2016) Hydrothermal liquefaction of spent coffee grounds in water medium for bio-oil production. Biomass Bioenergy 86:191–198
Yang J, He Q, Yang L (2019) A review on hydrothermal co-liquefaction of biomass. Appl Energy 250:926–945
Yin S, Dolan R, Harris M, Tan Z (2010) Subcritical hydrothermal liquefaction of cattle manure to bio-oil: effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour Technol 101(10):3657–3664
Zhang Y (2010) Hydrothermal liquefaction to convert biomass into crude oil. Bio Agric Waste Bioprod. https://doi.org/10.1002/9780813822716.ch10
Zhang S, Liu G (2021) Design and performance analysis of a hydrogen liquefaction process. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-021-02078-z
Zhang L, Xu C, Champagne P (2010) Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers Manage 51(5):969–982
Zhong C, Wei X (2004) A comparative experimental study on the liquefaction of wood. Energy 29(11):1731–1741
Zhou D, Zhang L, Zhang S, Fu H, Chen J (2010) Hydrothermal liquefaction of macroalgae enteromorpha prolifera to bio-oil. Energy Fuels 24(7):4054–4061
Zhu Z, Rosendahl L, Toor SS, Yu D, Chen G (2015) Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation. Appl Energy 137:183–192
Acknowledgements
This work was supported by the Thailand Science, Research & Innovation (TSRI) office (contract no. TSRI/fbr/640006) and Chiang Mai University (contract no. CMU/coe2563/eet).
Funding
Thailand Science, Research & Innovation (TSRI) office and Chiang Mai University.
Author information
Authors and Affiliations
Contributions
RS contributed to investigation, formal analysis, writing: original draft; TO contributed to methodology, investigation, formal analysis, writing: original draft; SP contributed to methodology, formal analysis; NT contributed to conceptualization, resources, supervision, writing: review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Informed Consent
All authors are fully aware and agree to this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saengsuriwong, R., Onsree, T., Phromphithak, S. et al. Conversion of tobacco processing waste to biocrude oil via hydrothermal liquefaction in a multiple batch reactor. Clean Techn Environ Policy 25, 397–407 (2023). https://doi.org/10.1007/s10098-021-02132-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-021-02132-w