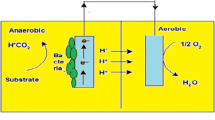
Abstract
A microbial fuel cell is a rapidly growing, eco-friendly and green technology. As per this technology, the microorganisms are employed to convert the chemical energy stored in the biodegradable portion of organic matter into direct electric current by simultaneously treating the wastewater. In this study, dual-chambered H-type mediator-less and membrane-less microbial fuel cell was operated and was optimized using synthetic wastewater as a substrate. The influence of various factors such as cathodic electron acceptors, electrode configuration, electrode spacing on chemical oxygen demand removal and current output were investigated. The maximum current of 1.72 mA was obtained using synthetic wastewater with potassium permanganate as effective catholyte, electrode spacing of 2 cm from the salt bridge and surface area of 98 cm2. This study also investigated the effect of substrate in the optimized MFC by applying different real wastewaters (municipal wastewater, dairy wastewater, cassava wastewater) and found a superior performance by dairy wastewater with maximum current output of 5.23 mA and chemical oxygen demand removal of 94%. Electron microscopic observations revealed the development of biofilm on the electrode surface, which was responsible for biocatalytic activity in the microbial fuel cell during the operation. The current generated using microbial fuel cell was supplied to peroxicoagulation process and was used for the removal of rhodamine B dye. Decolorization of 98% achieved by the novel microbial fuel cell-coupled peroxicoagulation system. The novel microbial fuel cell-coupled peroxicoagulation is an energy-efficient as well as cost-effective technique.
Graphic abstract

Similar content being viewed by others
Introduction
In recent years, the consumption of energy throughout the world has risen tremendously (Rahimnejad et al. 2015). Fossil fuels are the primary source of energy. However, due to adverse impact (global warming and pollution) on the environment, the consumption of fossil fuels as an energy source needs to be reduced (Slate et al. 2019). Another most crucial problem that needs immediate concern is the disposal of industrial effluents. Even though several conventional treatment techniques are available, the energy-intense operation and high sludge production constrain their applicability. Hence, there is a need for cost-effective alternative treatment technology.
Bioelectrochemical system (BES) is a complex system involving various techniques and concepts of microbiology, electrochemistry, electrical engineering, material science and environmental biotechnology (Patade et al. 2016). Microbial fuel cells, electrolysis cells, bio batteries and enzymatic biofuel cells are some of the applications of BES. The most commonly described BES is microbial fuel cells (MFCs). MFC is a developing technology which gives a potential solution for sustainable energy production by degrading the organic matter present in the wastewater. Thus, the production of direct current and wastewater treatment are simultaneously possible in MFC (Mustakeem 2015; Dong et al. 2015).
MFCs have many advantages, such as direct electric current generation, non-requirement of aeration, zero toxic byproducts formation, minimal sludge generation, self-regeneration of microorganisms, water reclamation, valuable products recovery, insignificant carbon foot print and lower operation cost. Thus, MFC not only has application in the field of wastewater treatment and bioelectricity generation, but it is also applicable in the field of biohydrogen production, biosensor and biorecovery (Mansoorian et al. 2014).
The construction and optimum operation of microbial fuel cells require a variety of tools from the disciplines of engineering, chemistry and biology (Mansoorian et al. 2014; Rajeswari et al. 2016). Several studies have reported the use of MFC with membrane (Ghasemi et al. 2015), without membrane (Logan et al. 2007), with mediator, without mediator (Sevda and Sreekrishnan 2012) and with biocathodes (Gonzalez del Campo et al. 2014) to improve the performance of MFC.
MFCs which use pure compounds like acetate or butyrate (Hidalgo et al. 2016), alcohol, fatty acid, monosaccharide sugar (Asensio et al. 2016), sucrose, acetate and glucose as substrate have been documented in the literature. Additionally, substrates like chemical wastewater (Venkata Mohan et al. 2008), dairy wastewater (Porwal et al. 2015), soak liquor (Sawasdee and Pisutpaisal 2016), dye industry wastewater (Kalathil et al. 2012; Patade et al. 2016), starch processing wastewater (Quan et al. 2014), leachate (Damiano et al. 2014), sugar mill effluent (Kumar et al. 2016), along with recent advances in the use of various substrates (Pandey et al. 2016), have been studied. The electrodes are one of the essential components of MFC, as bioelectrochemical oxidation and reduction reactions take place on the surface of electrodes. Thus, the performance of MFC depends on the electrode material. Hence, the electrode has to be selected in such a way that it must possess properties like high conductivity, excellent chemical stability, biocompatibility and cost effectiveness. The carbon-based electrode is preferred since metallic electrodes might be toxic to the microorganism. The dimensions, maximization of surface area and positioning of the electrode are some of the key considerations while choosing the electrodes (Butti et al. 2016). Another important component of MFC is membrane. Membranes allow the protons to flow from anode to cathode and insulate the transport of electrons through it. The major drawbacks of the process using membrane are its high cost, membrane fouling and constrained proton transfer. Similarly, electrons that are produced at the anode by the oxidation of fuel cannot be transferred directly from microbial cells to electrode due to the non-conductive nature of the cells. Therefore, to overcome the aforesaid problem, mediators were used. Generally, mediators are expensive as well as toxic phenolic substances. Hence, the long-term application of mediated MFCs for electricity production cannot be implemented at commercial scale. Furthermore, electrochemically active bacteria can be employed instead of mediators to transfer electrons to electrodes (Pankratov et al. 2015).
To overcome the constraints of conventional MFC system and to make the treatment system cost effective, researchers have extended their research in integrating the MFC system with other processes such as constructed wetland–MFC (Yadav et al. 2012), MFC–membrane bioreactor (Wang et al. 2012) and MFC–electro-Fenton (Zhu and Logan 2013).
Generally, in electro-Fenton (EF) process Fe2+ gets added externally as a catalyst, but in peroxicoagulation process sacrificial iron is used as an anode to produce Fe2+ in the solution, instead of external addition as in EF process (Brillas et al. 2003). Hence, peroxicoagulation system is the modified form of the EF system (Nidheesh and Gandhimathi 2014).
The sacrificial iron anode used in peroxicoagulation process undergoes oxidation and releases Fe2+ into the solution, thus supplying soluble Fe2+ to the process as in Eq. (1). At the same time, oxygen at the cathode surface undergoes reduction reaction with the acceptance of two electrons released from anode under acidic conditions, thus generating hydrogen peroxide as in Eq. (2). Hydroxyl radicals are generated in the electrolytic cell due to the Fenton reaction between in situ generated hydrogen peroxide and ferrous ion as in Eq. (3). The Fenton reaction produces highly oxidative hydroxyl radical, which helps in degradation of pollutant. Peroxicoagulation mechanism depends mainly on pH. Increase in pH of the solution with increase in electrolysis time is one of the specialties of peroxicoagulation process. At the above condition, pollutants are removed from aqueous phase by coagulation, not by degradation. Thus, peroxicoagulation process is a combination of EF and electrocoagulation process, as reported by several researchers (Nidheesh and Gandhimathi 2014; Venu et al. 2016)
With this background, the present study was carried out in a two-chambered H-type mediator-less and membrane-less MFC. MFC was optimized using synthetic wastewater having acetate as the sole carbon source with mixed bacterial cultures. The influence of various operational parameters such as cathodic electron acceptors, electrode spacing and electrode configuration on Chemical Oxygen Demand (COD) removal and current output were studied. Later, various substrates (municipal wastewater, dairy wastewater, cassava wastewater) were tested with the optimized MFC, and the effect on current output and COD removal was evaluated. This study also attempted to integrate the optimized dual-chambered MFC with peroxicoagulation process for the dye removal.
Materials and methods
Materials
The various chemicals needed for the study were obtained from Merck. Synthetic wastewater was prepared with the following composition: sodium acetate—1.61 g L−1, potassium dihydrogen phosphate—0.2 g L−1, dipotassium hydrogen phosphate—1.06 g L−1, magnesium sulfate—0.5 g L−1, calcium chloride—0.05 g L−1, potassium nitrate—2 g L−1, sodium chloride—1 g L−1 whose initial COD was around 1000 mg L−1. Activated sludge from the sewage treatment plant in National Institute of Technology, Tiruchirappalli, Tamil Nadu, India, was used as an inoculum. The inoculum had a mixed liquor suspended solids (MLSS) concentration of 5500 mg L−1; sludge volume index (SVI) of 110 mL g−1; mixed liquor volatile suspended solids (MLVSS) concentration of 4125 mg L−1 and MLVSS to MLSS ratio of 0.75. For MFC-coupled peroxicoagulation experiments, rhodamine B (RhB) (chemical formula, C28H31ClN2O3) purchased from Loba Chemie was used. An aqueous solution was prepared with the composition of 10 mg L−1. The rectangular graphite plate used as an electrode purchased from Anabond Sainergy Fuel Cell India Pvt. Ltd, Chennai. The iron plate procured from the local market in Tiruchirappalli was used as an anode in peroxicoagulation process.
Collection and characterization of wastewater
To determine the effect of various substrates on the performance of MFC, the experiments were conducted using three different types of wastewater (municipal wastewater, dairy wastewater and cassava wastewater). The municipal wastewater (MW) was collected from the sewage treatment plant in National Institute of Technology, Tiruchirappalli, Tamil Nadu, India. Dairy wastewater (DW) was rich in organic matter and carbohydrate and collected from Aavin milk processing firm, Tiruchirappalli. The cassava wastewater (CW) was also rich in carbohydrates and starch collected from tapioca products industry, Salem. The characterization of all the wastewaters was done according to APHA standards and is provided in Table 1. The samples were stored at 4 °C in the refrigerator for further analysis.
Experimental procedure
The present study was carried out in two stages. In the initial stage, electricity generation via MFC was tested by considering various industrial wastewaters as substrate. The optimized MFC condition was used for the second stage of experiments. In this stage, MFC was coupled with peroxicoagulation process and tested for its ability to remove dyes from water medium.
Microbial fuel cell
The reactor (anodic and cathodic chambers) was made using air-tight food-grade plastic containers of volume 1 L constituting a working volume of 800 mL. In both the plastic containers, a side opening of 1.4 cm diameter was made at the height of 3/4th of each container from the bottom. Both anodic chamber and cathodic chamber were connected using a glass rod (10 cm Length × 1.25 cm diameter) containing agar mixture (7% agar and 10% KCl) (Patade et al. 2016) which will act as a salt bridge, thus allowing the protons to travel. Copper wires were used for connecting the electrodes with an external resistance of 10 Ω and were sealed using epoxy resin. All the electrodes were thoroughly washed with distilled water to remove any debris and then soaked in 1 N HCl for one hour followed by 1 M NaOH for another 1 h before the start of every experiment to avoid possible biomass contamination.
Microbial fuel cell setup was assembled with the pretreated electrode in the adjoining chambers. The MFC was operated in batch mode at room temperature; activated sludge was inoculated with the synthetic wastewater in the ratio of 1:3 and was used as an anolyte with a pH of 5.8–6 (acidophilic environment) (Sevda and Sreekrishnan 2012; Venkata Mohan et al. 2008). The pH of the catholyte was maintained to be neutral. The electrodes were kept 5 cm from the salt bridge in both anodic and cathodic chambers and the distance between electrodes fixed as 20 cm. The performance of MFC was evaluated by estimating the COD removal efficiency, and the voltage (V) measurements were recorded daily using digital multimeter by connecting 10 Ω external resistances.
Morphological features of biofilm organisms in the MFC were determined using scanning electron microscopy (SEM) analysis. If the biofilm samples were directly dried, the bacterial morphology would collapse. Hence, before SEM analysis, bacterial fixation was done. Initially, biofilm-loaded samples (anode was taken out from the chamber) were immersed in 5% of formaldehyde solution for 3 h to fix the bacterial cells. Then, the samples were dehydrated stepwise in a graded series of ethanol solutions (25, 50, 70, 85, 95 and 100%) and then dried (Zhang et al. 2009). The samples were then sputter-coated in a sputter coater and observed in SEM.
Influence of electrode configuration
To study the impact of various anodic configurations on the performance of MFC, the experiments were conducted with plain and perforated graphite plates. The perforated electrode was made by providing 16 holes of 0.5 cm diameter, which increases the surface area from 82 cm2 (plain graphite plate area) to 88 cm2. Additionally, 25 holes of 0.4 cm increased the surface area to 93 cm2. Similarly, the addition of 13 holes of 0.4 cm increased the surface area to 98 cm2. Then, the variation in electric potential and COD with different configurations was measured.
MFC-coupled peroxicoagulation process
Peroxicoagulation experiments were carried out in a reactor; graphite plate (effective area 25 cm2) was used as the cathode, and iron plate (effective area 25 cm2) was used as the anode. Electrodes were kept at an inter-electrode distance of 2 cm. The pH of the RhB aqueous solution (600 mL) was adjusted to 3 using 0.5 N H2SO4. Air was bubbled through fish aerator to supply oxygen to the system. The current generated from the MFC set up was supplied to the electrolytic cell. The dye removal efficiency, change in solution pH, and the iron leaching in the aqueous solution was determined. RhB concentration was determined using UV/Vis spectrophotometer (Lambda 25, PerkinElmer, USA) at the peak wavelength of 553.8 nm. The sludge produced in the peroxicoagulation process was analyzed using Fourier transform infrared (FTIR) and X-ray diffraction (XRD) analysis.
Results and discussion
Effect of cathodic electron acceptors
The electric potential between the anode and the cathode influences the power generation. The cathodic electron acceptor of high redox potential is highly desirable for the operation of MFC. A series of experiments were conducted with different cathodic electron acceptor to study the performance of MFC. In this study, aerated catholyte (DO-6.7 mg L−1), potassium permanganate (0.2 M) and potassium ferricyanide (0.2 M) were used as catholyte; whereas, synthetic wastewater was used as an anolyte. The variation in current production with respect to different cathodic acceptors in MFC is shown in Fig. 1a.
a Variation of current with time under batch process using different cathodic electron acceptors, b pH transition at the end of fuel cell operation in the catholyte effect (experimental conditions: electrode—plain graphite plate (82 cm2), electrode spacing—5 cm from salt bridge, anolyte—synthetic wastewater, pH at anode—5.5–6, cathode—neutral)
Due to the lag phase, initially there was less current production. Followed by the lag phase, there was an increase in cell current. This was due to the presence of easily degradable components in the wastewater, which was utilized by the mixed microorganisms. When these substrates were completely used up, there was a decrease in current output. After which complex substrates present in the wastewater were taken up by microorganisms by which lower current generation was still obtained (Mathuriya and Sharma 2009). The study was conducted initially with DO based aerated catholyte, the cathodic chamber was filled with normal distilled water of DO-6.7 mg L−1 and was continuously purged with air using fish tank aerator to increase the oxygen solubility of cathode.
The maximum voltage of 4.7 mV and the current output of 0.47 mA was obtained on 5th day of MFC using synthetic wastewater as fuel and aerated catholyte. Maximum COD Removal efficiency of aerated catholyte was 83.8%. Similarly, on the 5th day of MFC, the maximum current output of 0.63 mA and 0.52 mA was obtained using potassium permanganate (0.2 M) and potassium ferricyanide (0.2 M) as catholyte, respectively. From Fig. 1a, during initial hours the current output profile, shows an increase in current with potassium ferricyanide which later decreased, this might be due to some columbic loss or bacterial metabolic loss in the system. Similarly, after 50th hour current output in potassium permanganate was showing increase in current production, this might be due to effective oxygen reduction at the cathode side. The COD removal efficiency of 86.7% and 84.2% was achieved using potassium permanganate and potassium ferricyanide, respectively. The high COD removal efficiency by potassium permanganate is due to its high redox potential (You et al. 2006) (EO = 1.70 V where the permanganate accepts the electrons and gets reduced to manganese dioxide) when compared to ferricyanide and oxygen. Hence higher the potential difference between anode and cathode resulted in higher electron consumption at the cathode. Also, the significant advantages of potassium permanganate are cost-effectiveness and environment-friendly performance. Previous studies have also reported potassium permanganate as an effective cathodic electron acceptor. You et al. (2006) obtained a maximum power density of 115.60 mWm−2 with permanganate as the cathodic electron acceptor, whereas hexacyanoferrate produced 25.62 mWm−2 and oxygen of 10.2 mWm−2 as the cathodic electron acceptor in a two-chamber MFC. Patade et al. (2016) have found that the maximum current output of 0.64 mA using potassium ferricyanide in comparison with various electron acceptors (potassium permanganate, potassium dichromate, and potassium ferricyanide and potassium chromate). Though potassium ferricyanide acts as an excellent electron acceptor, it has certain limitations like chemical instability, production of toxic hydrocyanic acid and the expense. Thus, the results obtained in the present study suggest that the cathodic reaction in MFC has a serious limiting factor to be considered, as the oxygen reduction in the cathodic chamber is coupled with substrate degradation in the anodic chamber.
Also, the pH transition during MFC operation was studied, which is represented in Fig. 1b. Since pH is a crucial parameter for power output, pH was noted before and after the MFC operation. The pH of the synthetic wastewater was adjusted to 6, 5.9, 5.7, whereas the pH of catholyte (KMnO4, K3FeCN6 and aerated water) solution was adjusted to neutral pH. At the end of the operation, pH of the wastewater increased to 7.3, 7.1, 7.5 and pH of the catholyte (KMnO4, K3FeCN6 and aerated water) increased to 9, 10.5, and 8.3, respectively. The results clearly showed that the proton diffusion and migration through salt bridge was effective on subject to the accumulation of protons causing acidification at the anode. Similarly, on the cathode side alkalization occurred, this is due to oxygen reduction reaction with the continuous acceptance of electrons and protons, thus increases the current output. In the cathode side, potassium ferricyanide shows more pH variation than other catholytes; this may be due to effective reduction reaction occurrence during the fuel cell operation. Though the reaction progressed effectively, the current production was not so high when compared to potassium permanganate, this lesser current production in ferricyanide might be owed to the occurrence of alternate reactions which may have inhibited the flow of electrons resulting in low current generation. In case of KMnO4 catholyte, the high pH difference was observed between anolyte and catholyte solution when compared to other catholytes. This increased the proton flux rate between the anode and cathode chamber solutions and resulted in more current production (Jadhav and Ghangrekar 2009).
Effect of electrode spacing
A series of experiments were conducted to study the effect of electrode spacing on the current output. The electrodes kept at different spacings such as 1 cm, 2 cm, 4 cm, 5 cm and 6 cm from the salt bridge. When the distance between the electrodes is small, the travel distance for protons and electrons is also short, thus enhancing the performance of microbial fuel cell. The present study showed a maximum current output of 0.99 mA when the inter-electrode distance was maintained at 2 cm from the salt bridge, whereas current of 0.82 mA, 0.71 mA, 0.63 mA and 0.56 mA was produced when the inter-electrode distance was maintained at 1 cm, 4 cm, 5 cm and 6 cm from salt bridge, respectively, as shown in Fig. S1. This might be due to the reason that when electrodes are closer to the salt bridge, the proton movement is facilitated effectively, thereby reducing the internal resistance leading to an increase in current generation. It has been reported that the internal resistance would be low as the electrode spacing is small (Li et al. 2011). Further, reduction in inter-electrode distance to 1 cm, which leads to oxygen diffusion into the anode chamber, might be the reason for lesser current production, when compared to 2-cm inter-electrode spacing. Zhang et al. (2013) reported that the diffusion of oxygen into an anode chamber affects the anaerobic condition and leads to deterioration of MFC performance.
Similarly, the COD reduction efficiency was more for the inter-electrode spacing of 2 cm from salt bridge when compared to spacing 1 cm, 4 cm, 5 cm and 6 cm. The efficiency reached 91.6% for 2 cm, whereas 1 cm, 4 cm, 5 cm and 6 cm showed the COD removal efficiency of 90%, 89%, 86.7% and 84%, respectively. When the electrode distance was less, rapid anodic reactions occur. This might be the reason for achieving high COD removal efficiency.
Effect of electrode configuration
Generally, the current generation in MFC depends on the bacterial cell concentration on the electrode surface area. Anode material and its configuration also play a significant role in the electron transfer mechanism. To study the mechanism, various types of anode materials such as plain graphite, carbon paper, carbon cloth, carbon felt, RVC (reticulated vitreous carbon) or graphite granules were used. The electron transfer from the bacterial cells to the anode is mainly dependent on the physical contact between the cells and electrode.
The different perforated and plain graphite electrodes were operated in batch mode with synthetic wastewater having COD of 1000 mg L−1, potassium permanganate as catholyte and an electrode spacing of 2 cm. On the 6th day of the operation, the maximum current obtained for MFC with the plain electrode (82 cm2) was 0.99 mA. Similarly, the maximum current obtained for MFC with a perforated electrode of 88 cm2 was 1.22 mA, that for 93 cm2 was 1.65 mA, and that for 98 cm2 was 1.72 mA as shown in Fig. S2. This effective electron transfer is due to the acquisition and adaptation of microorganisms over the perforations on the anode surface. Similarly, the COD removal efficiency of plain graphite plate (82 cm2) was 91.6%, that of perforated graphite plate (88 cm2) was 93%, that of perforated graphite plate (93 cm2) was 95.6%, and that of perforated graphite plate (98 cm2) was 97.2%.
The maximum current output was obtained in a graphite electrode of 98 cm2. This is because the extended porosity of the perforated graphite plate promotes the high surface area, thus providing more thriving space for the microorganism to uproot on the anodes, and facilitates the effective electron transfer.
The coulombic efficiency (CE) was calculated using Eq. (4) reported by Jadhav and Ghangrekar (2009)
where CE = measured current; CT = theoretical current production. The measured current and theoretical current are estimated using Eqs. (5) and (6), respectively,
where I = current; t = time duration (h); F = Faraday constant (96485 C/mol); n = no. of moles of electrons produced per mole of substrate, n = 4 for wastewater COD, W = daily COD load removed in gram; M = molecular weight of substrate, g/mol.
The coulombic efficiency (CE) of 5.6%, 8.16%, 13.42% and 13.76% was observed for an electrode configuration of 82 cm2, 88 cm2, 93 cm2 and 98 cm2, respectively. Coulombic yield increases with increase in electrode surface area. Bond and Lovley (2003) had stated that the electrode surface area was also one of the influencing parameters that affects the current output. Ghangrekar and Shinde (2007) studied the effect of electrode configuration by placing single, double, triple electrodes of different electrode surface area (70.21 cm2, 140.43 cm2 and 210.64 cm2, respectively) in the anode chamber. However, the author did not find any significant change in the voltage with respect to change in the electrode configuration. Liu and Li (2007) found that there was an increase in voltage (from 0.14 to 0.36 V) when the anode surface area was increased (26 cm2 to 65 cm2). This increase in voltage is due to more microbial attachment in the larger anode surface area, thereby increasing the electron transfer rate. The present results prove that higher anode surface area can significantly improve the current production which may be quantitatively dependent on the biofilms attached on the electrode area. As described earlier, pH transition at the end of operation was studied and the results were similar, and the pH increased at the end of operation in both anodic and cathodic chambers.
Influence of substrate in optimized MFC
For a simple molecule, the process is easy and it follows a different pathway, whereas for the complex molecules the degradation and metabolic pathway vary. Similarly, the electrolyte pH plays a significant role in MFCs power output because the pH of the electrolyte should be near to the optimal pH for favorable growth of bacteria. Hence, it is necessary to find out the optimal working pH for all the wastewaters. The dual-chambered H-type MFC made of food-grade plastic container was operated with different wastewaters as substrate (anolyte) and potassium permanganate (0.2 M) as catholyte with 98 cm2 electrode surface area having 2-cm electrode spacing from the salt bridge. The system was operated in batch mode with room temperature. The anodic chamber of MFC-1 was fed with 800 mL of municipal wastewater with 720 mg COD. L−1 (low strength); MFC-2 was fed with 800 mL of dairy wastewater with 10,400 mg COD L−1 (high strength), and MFC-3 was fed with 800 mL of cassava wastewater of 5,400 mg COD L−1 (moderate strength), where the native microorganisms (mixed culture) present in the wastewater act as inoculum. The experiment was carried out with four MFC-1 setups with different anolyte pH (5, 6, 7, 8) and potassium permanganate as catholyte whose pH was adjusted to neutral. Similarly, four MFC-2 and MFC-3 setups were made with different pH ranges. All the experiments were conducted simultaneously using four separate dual-chambered MFC for MFC-1, MFC-2 and MFC-3. At each anodic pH, the MFC was operated for 7 days in the ambient room temperature. At the end of 5th day in MFC-1 (municipal wastewater), maximum current output of 2.74 mA was obtained when the pH was maintained at 7, whereas current of 0.73 mA, 1.61 mA and 0.92 mA was obtained when the pH was maintained at 5, 6 and 8, respectively, as shown in Fig. S3a. Similarly, the COD removal efficiency was more for pH 7 as compared to other pH. The efficiency reached 90% for pH 7, whereas pH 5, 6 and 8 showed the COD removal efficiency of 67%, 85% and 70%, respectively, as shown in Fig. 2a. The present study demonstrates the optimal pH for the municipal wastewater as 7 in the MFC operation and a significant variation in current generation and COD removal efficiency of MFC with other pH values.
In MFC-2 at the end of 6th day, a maximum current output of 5.23 mA was obtained when the pH was maintained at 7, whereas current of 0. 95 mA, 3.38 mA and 1.47 mA was obtained when the pH was maintained at 5, 6 and 8, respectively, as shown in Fig. S3b. Similarly, the COD removal efficiency was more at pH 7 as compared to other pH. The efficiency reached up to 94% for pH 7, whereas pH 5, 6 and 8 showed the COD removal efficiency of 77%, 90% and 83%, respectively, as shown in Fig. 2b.
In MFC-3 at the end of 7th day, a maximum current output of 1.93 mA was obtained when the pH was maintained at 8, whereas current of 0.89 mA, 0.91 mA and 1.12 mA was obtained when the pH was maintained at 5, 6 and 7, respectively, as shown in Fig. S3c. Similarly, the COD removal efficiency was more at pH 8 as compared to others. The efficiency reached 61% at pH 8, whereas pH 5, 6 and 7 showed the COD removal efficiency of 37%, 40% and 53%, respectively, as shown in Fig. 2c. The result of this study (cassava wastewater) showed that microbial activity is slower in pH less than and greater than the optimum pH (i.e., 8). Low pH in the anode chamber deactivates microorganisms and decreases the MFC efficiency (Agarry et al. 2016). The optimum pH obtained for municipal wastewater and dairy wastewater is 7, whereas for cassava wastewater, the optimum pH is 8. Agarry et al. (2016) studied the effect of pH in cassava mill effluent and reported that the maximum efficiency of MFC is attained in pH 8, which is correlated with this study. Elakkiya and Matheswaran (2013) studied the effect of pH using dairy wastewater and found that the optimal pH is 7. However, the microbial activity is slower in sub-optimal pH. Jadhav and Ghangrekar (2009) found that the highest current was generated at pH of 6.5 in the anodic chamber. Also, the current was lower when the pH of influent to the anode chamber was more than 7.0 and less than 6.0. Higher pH difference between anodic and cathodic electrolytes favored higher current and voltage.
From the present study, it was observed that the pH, the substrate and its concentration are also the crucial parameters that affects the performance of MFC. The time taken by the microorganism to reach the equilibrium (steady state) with different substrates and its concentration would be different. Though municipal wastewater is a mixture of complex compounds, owing to its low strength it degraded effectively with 90% COD removal efficiency and the current output of 2.74 mA meanwhile, dairy wastewater (proteinous) which had high strength of biodegradable substance also degraded effectively with maximum COD removal of 94% due to presence of dissolved proteins, fats and sugars which are good source of nutrition for microorganisms (Mathuriya and Sharma 2009). However, cassava wastewater of moderate strength did not give much efficiency when compared to other substrates. This might be due to the presence of some other complex substances or starch and cellulose content in the wastewater that may inhibit the operation and because the major waste materials obtained from cassava are the outer brown peel that contains lignified cellulosic material and the inner white portion of parenchymatous material which has toxic cyanogenic glucosides. Mathuriya and Sharma (2009) found that because of its insolubility and chemical stability that limits the rate of substrate degradation, cellulose and cellulosic wastes are not ideal substrates for electricity generation in MFCs. Similar studies have been carried out in previous literatures as well. Mathuriya and Sharma (2010) obtained a maximum current output of 14.92 mA and 90.23% COD removal in 10-day operation of MFC for beer brewery wastewater in comparison with sugar industry wastewater, dairy wastewater, municipal wastewater and paper industry wastewater. Nimje et al. (2012) compared four different real-field wastewaters (agricultural wastewater, domestic wastewater, paper wastewater and food/dairy wastewater) using pure strain and mixed cultures. Food/dairy wastewater gave high substrate degradation and columbic efficiency. Asensio et al. (2016) evaluated the influence of fuel and its dosage in double-chambered MFC. Five types of fuels (ethanol, glycerol, acetate, propionate and fructose) were studied. Carboxylic acids were more effective than alcohols and fructose. The characterization of all the wastewaters after MFC operation is shown in Table 2. The post-treatment pH of all wastewater was neutral. There was a significant turbidity removal in MW (93.1%), DW (82%) and CW (63%). The total solids removal efficiency was 32% (MW), 60% (DW) and 35% (CW). BOD reduction in MW, DW and CW was 81%, 96% and 81%, respectively. By using synthetic wastewater (acetate as sole carbon source of 1000 mg COD L−1), the organic matter removal was 97.2% with the maximum current output of 1.72 mA which revealed that the substrate and its concentration and the type of microorganisms present are also important parameters that need to be considered in the MFC study.
SEM analysis of anode biofilm
Morphological features of biofilm organisms in the MFC were determined using SEM analysis as shown in Fig. S4. In the synthetic wastewater-fed MFC, the bacterial examination revealed that the rod-shaped microbial clumps were scattered in the electrode surface and proved that the increase in microbial populations and a diverse microbial community was enriched during the MFC operation and also nanowires were not found. The SEM image of municipal wastewater-fed MFC (MFC-1) shows a similar type of result in acetate-fed MFC. The rod-shaped bacteria were found, and there was no nanowire formation. Previous studies also propose similar results. Gregory et al. (2004) demonstrated through SEM analysis that when the electrodes were inoculated with the river sediment, the results were similar to the present study. The authors suggested the mechanism of direct electron transfer where the electrons are transported by bacteria using diverse respiration strategies and by directly getting attached to the electrode (anode) which would be carried to the cathode via external wire. Bond and Lovley (2003) claimed that a dense community of organisms were coated on the electrode surface. Also, Geobacteraceae is known for the direct attachment of the cells to the electrode. Different morphological features of the biofilm like pilus like surface appendages (nanothread) (Patade et al. 2016), the loosely attached clumps of bacteria, rod-shaped fusiform bacteria (Ghangrekar and Shinde 2007) and heterogeneous nature (Rajeswari et al. 2016) were also observed. The SEM image of the dairy wastewater-fed MFC (MFC-2) shows the formation of rod-shaped bacillus in the system. The bacterial adhesion in the anode was high which proved that the increase in microbial population and a diverse microbial community. Mardanpour et al. (2012) studied the morphological features of biofilm formed using dairy wastewater and demonstrated a good bacterial adhesion in the spiral anode. The SEM image of the cassava wastewater-fed MFC (MFC-3) shows that the formation of spherical-shaped bacteria (cocci) tightly linked to one another. These organisms are enriched in the biofilm.
MFC-coupled peroxicoagulation process
The optimized dual-chambered MFC was taken with dairy wastewater as anolyte since it gave maximum current output. Peroxicoagulation experiments were carried out using rhodamine B of 10 mg L−1 aqueous solution. It was observed that the dye decolorization was very effective (Fig. 3). The MFC generates the maximum current output of 5.23 mA. It was observed from Fig. 3 that the dye removal efficiency increased with increase in time. Higher removal efficiency was obtained in the initial stages of electrolysis. Nearly 67% of RhB was removed in 1 day of electrolysis. The decolorization was nearly 96% at the end of 4th day of process and almost 98% at 8th day of the process. This increase in RhB removal rate at initial stages is due to the presence of high concentration of RhB, and hence, there is higher possibility for the collision between dye molecules and the hydroxy radicals. As time progresses, after 4th day the removal rate slows down due to lower available concentration of RhB (Nidheesh and Gandhimathi 2014). The daily RhB spectra variations were monitored using UV spectroscopy (Fig. S5). It was observed that the peak at 553.8 nm was lowered and the spectrum was broadened. At the end of 2nd day itself, the peak at 553.8 nm vanished almost which shows that RhB removal happened effectively using MFC coupled with peroxicoagulation process.
The change in solution pH is the important aspect that the Fenton-related mechanism is greatly dependent on pH. Fenton-related processes was operated successfully in the pH near to 3 (Nidheesh 2015). The solution pH 3 favors the production of hydroxyl radical and the removal of pollutant by mineralization, whereas pH > 3 favors the removal of pollutant by in situ generated coagulants ([Fe2(H2O)8(OH)2]4+, [Fe2(H2O)7(OH)3]3+ and [Fe2(H2O)7(OH)4]2+) rather than mineralization.
The daily variation in solution pH was monitored, and it was found that the solution pH was increasing daily. In the 1st day of the electrolysis itself, the pH increased to 4.78 with 67% color removal efficiency. The percentage decolorization of RhB in peroxicoagulation study and the change in solution pH with different electrolysis time are also shown in Fig. 3. This continual increase in pH with time is due to the hydroxyl radical produced in the solution which was not compensated by the water oxidation. For Fe–H2O system, for pH < 3.5 the solubility equilibrium was controlled by Fe3+. During this case, in comparison with other processes Fenton process occurs effectively. For the solution pH > 3.5, Fe(OH)3 controls the solubility equilibrium than Fe3+. During this case, the formation of ferric hydroxide flocs was responsible for the removal of dye molecules by sorption or coagulation process (Nidheesh et al. 2013). Hence, in the present study, the mechanism of RhB removal is due to electrocoagulation process in the electrolytic system.
For the effective hydroxyl radical production in the solution, it is also necessary to determine the sufficient concentration of iron in the solution. Hence, the total iron concentration in the effluent was noted daily (Fig. 3). The iron concentration increased up to 1.854 mg L−1. The production of iron sludge was observed in the peroxicoagulation process. The maximum iron sludge of 45 mL was obtained. The characterization of sludge was carried out using FTIR and XRD analyses.
FTIR spectrum of the sludge produced in the peroxicoagulation study is shown in Fig. 4a. The peaks are found near 3430, 2830, 1596 and 1020 cm−1. The broad band found at 3430 cm−1 could be due to the stretching vibrations of O–H groups. The peak found at 2830 cm−1 could be due to the presence of aliphatic groups (–CH, –CH2– and –CH3). The strong band spectra at 1596 cm−1 might be due to the presence of stretching vibrations of unsaturated aliphatic, aromatics, unsaturated heterocycles (C = C) groups. The bands near 1020 cm−1 could be due to the stretching in plane vibration of lepidocrocite (γ-Fe–O–OH) (Namduri and Nasrazadani 2008).
The XRD pattern of the sludge produced in peroxicoagulation study is shown in Fig. 4b. The peaks were observed at 2Ө = 23.24°, 27.05°, 36.01°, 47.09°, 52.94° and 67.35°. The peaks observed at 27.05° and 36.01° were due to the presence of iron oxide hydroxide (FeOOH) (JCPDS File No. 896096). The peaks observed at 47.09°, 52.94° were also due to the presence of FeOOH (JCPDS File No. 762301). The peaks observed at 23.24° and 67.354° were due to the presence of Fe2O3 (JCPDS File No. 895894). This indicates that RhB is removed from aqueous solution by degradation via Fenton reactions and separation via electrocoagulation process.
In real-field applications, pH of the system shall be regulated to pH 3 at every 10 min to avoid sludge formation. Even the sludge produced during the peroxicoagulation process is rich in Fe content, which can be reused as a heterogeneous Fenton catalyst (Nidheesh and Gandhimathi 2014).
Conclusion
Dual-chambered H-type mediator-less and membrane-less MFC was inoculated with activated sludge and was operated successfully. Among the different catholytes used in the study (KMnO4, K3FeCN6 and aerated water), KMnO4 showed maximum current output (0.63 mA) and COD removal efficiency (86.7%). An electrode distance of 2 cm from salt bridge is effective for achieving maximum current and as well as COD removal efficiency. Perforated electrode with 98 cm2 showed a maximum current output of 1.72 mA. Then, for optimized MFC, the effect of fuel was studied with various real-field wastewaters. The results showed that dairy wastewater produced the maximum current output of 5.23 mA at pH 7 with the COD removal efficiency of 94% and municipal wastewater produced the maximum current output of 2.61 mA at pH 7 with the COD removal efficiency of 90% and the cassava wastewater produced the maximum current output of 1.93 mA at pH 8 with the COD removal efficiency of 61%. SEM images proved the formation of biofilm which facilities the direct electron transfer mechanism, thus generating bioelectricity. An attempt was made to integrate MFC coupled with peroxicoagulation. The current generated using MFC was supplied to peroxicoagulation process and observed 98% decolorization of RhB. The use of MFC for providing power to peroxicoagulation process can be an efficient method for pollutant degradation.
References
Agarry SE, Oghenejoboh KM, Solomon BO (2016) Bioelectricity production from cassava mill effluents using microbial fuel cell technology 35:329–336. https://doi.org/10.4314/njt.v35i2.13
Asensio Y, Fernandez-Marchante CM, Lobato J, Canizares P, Rodrigo MA (2016) Influence of the fuel and dosage on the performance of double-compartment microbial fuel cells. Water Res 99:16–23. https://doi.org/10.1016/j.watres.2016.04.028
Bond DR, Lovley DR (2003) Electricity production by geobacter sulfurreducens attached to electrodes electricity production by geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69:1548–1555. https://doi.org/10.1128/AEM.69.3.1548
Brillas E, Boye B, Baños MÁ et al (2003) Electrochemical degradation of chlorophenoxy and chlorobenzoic herbicides in acidic aqueous medium by the peroxi-coagulation method. Chemosphere 51:227–235. https://doi.org/10.1016/S0045-6535(02)00836-6
Butti S, Velvizhi G, Sulonen MLK, Haavisto JM, Koroglu EO, Cetinkaya AY, Singh S, Arya D, Modestra JA, Vamsi Krishna K, Verma A, Ozkaya B, Lakaniemi AM, Puhakka JA, Venkata Mohana S (2016) Microbial electrochemical technologies with the perspective of harnessing bioenergy: maneuvering towards upscaling. Renew Sustain Energy Rev 53:462–476. https://doi.org/10.1016/j.conbuildmat.2013.12.054
Damiano L, Jambeck JR, Ringelberg DB (2014) Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl Biochem Biotechnol 173:472–485. https://doi.org/10.1007/s12010-014-0854-x
Dong Y, Feng Y, Qu Y, Du Y, Zhou X, Liu J (2015) A combined system of microbial fuel cell and intermittently aerated biological filter for energy self-sufficient wastewater treatment. Sci Rep 5:1–8. https://doi.org/10.1038/srep18070
Elakkiya E, Matheswaran M (2013) Comparison of anodic metabolisms in bioelectricity production during treatment of dairy wastewater in microbial fuel cell. Bioresour Technol 136:407–412. https://doi.org/10.1016/j.biortech.2013.02.113
Ghangrekar MM, Shinde VB (2007) Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour Technol 98:2879–2885. https://doi.org/10.1016/j.biortech.2006.09.050
Ghasemi M, Halakoo E, Sedighi M, Alam J, Sadeqzadeh M (2015) Performance comparison of three common proton exchange membranes for sustainable bioenergy production in microbial fuel cell. Procedia CIRP 26:162–166. https://doi.org/10.1016/j.procir.2014.07.169
Gonzalez del Campo A, Perez JF, Cañizares P, Rodrigo MA, Fernandez FJ, Lobato J (2014) Study of a photosynthetic MFC for energy recovery from synthetic industrial fruit juice wastewater. Int J Hydrog Energy 39:21828–21836. https://doi.org/10.1016/j.ijhydene.2014.07.055
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604. https://doi.org/10.1111/j.1462-2920.2004.00593.x
Hidalgo D, Tommasi T, Velayutham K, Ruggeri B (2016) Long term testing of microbial fuel cells: comparison of different anode materials. Bioresour Technol 219:37–44. https://doi.org/10.1016/j.biortech.2016.07.084
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100:717–723. https://doi.org/10.1016/j.biortech.2008.07.041
Kalathil S, Lee J, Cho MH (2012) Efficient decolorization of real dye wastewater and bioelectricity generation using a novel single chamber biocathode-microbial fuel cell. Bioresour Technol 119:22–27. https://doi.org/10.1016/j.biortech.2012.05.059
Kumar R, Singh L, Zularisam AW (2016) Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sustain Energy Rev 56:1322–1336. https://doi.org/10.1016/j.rser.2015.12.029
Li WW, Sheng GP, Liu XW, Yu HQ (2011) Recent advances in the separators for microbial fuel cells. Bioresour Technol 102:244–252. https://doi.org/10.1016/j.biortech.2010.03.090
Liu Z, Li H (2007) Effects of bio- and abio-factors on electricity production in a mediatorless microbial fuel cell. Biochem Eng J 36:209–214. https://doi.org/10.1016/j.bej.2007.02.021
Logan B, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 41:3341–3346. https://doi.org/10.1021/es062644y
Mansoorian HJ, Mahvi AH, Jafari AJ, Khanjani N (2014) Evaluation of dairy industry wastewater treatment and simultaneous bioelectricity generation in a catalyst-less and mediator-less membrane microbial fuel cell. J Saudi Chem Soc 20:88–100. https://doi.org/10.1016/j.jscs.2014.08.002
Mardanpour MM, Esfahany MN, Behzad T, Sedaqatvand R (2012) Single chamber microbial fuel cell with spiral anode for dairy wastewater treatment. Biosens Bioelectron 38:264–269. https://doi.org/10.1016/j.bios.2012.05.046
Mathuriya AS, Sharma VN (2009) Bioelectricity production from paper industry waste using a microbial fuel cell by Clostridium species. J Biochem Technol 1:49–52
Mathuriya SA, Sharma VN (2010) Bioelectricity production from various wastewaters through microbial fuel cell technology. J Biochem Technol 2:133–137
Mustakeem (2015) Electrode materials for microbial fuel cells: nanomaterial approach. Mater Renew Sustain Energy 4:1–11. https://doi.org/10.1007/s40243-015-0063-8
Namduri H, Nasrazadani S (2008) Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros Sci 50:2493–2497. https://doi.org/10.1016/j.corsci.2008.06.034
Nidheesh PV (2015) Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review. RSC Adv 5:40552–40577. https://doi.org/10.1039/C5RA02023A
Nidheesh PV, Gandhimathi R (2014) Electrolytic removal of Rhodamine B from aqueous solution by peroxicoagulation process. Environ Sci Pollut Res 21:8585–8594. https://doi.org/10.1007/s11356-014-2775-1
Nidheesh PV, Gandhimathi R, Ramesh ST (2013) Degradation of dyes from aqueous solution by Fenton processes: a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-012-1385-z
Nimje VR, Chen CY, Chen HR, Chen CC, Huang YM, Tseng MJ, Cheng KC, Chang YF (2012) Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour Technol 104:315–323. https://doi.org/10.1016/j.biortech.2011.09.129
Pandey P, Shinde VN, Deopurkar RL, Kale SP, Patil SA, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723. https://doi.org/10.1016/j.apenergy.2016.01.056
Pankratov D, Sundberg R, Sotres J, Maximov I, Graczyk M, Suyatin DB, González-Arribas E, Lipkin A, Montelius L, Shleev S (2015) Transparent and flexible, nanostructured and mediatorless glucose/oxygen enzymatic fuel cells. J Power Sources 294:501–506. https://doi.org/10.1016/j.jpowsour.2015.06.041
Patade S, Silveira K, Babu A, Mhatre Y, Saini V, Rajput R, Mathew J, Birmole R (2016) Bioremediation of dye effluent waste through an optimised microbial fuel cell. Int J Adv Res Biol Sci 3:214–226
Porwal HJ, Mane AV, Velhal SG (2015) Biodegradation of dairy ef fl uent by using microbial isolates obtained from activated sludge. Water Resour Ind 9:1–15. https://doi.org/10.1016/j.wri.2014.11.002
Quan X, Tao K, Mei Y, Jiang X (2014) Power generation from cassava alcohol wastewater: effects of pretreatment and anode aeration. Bioprocess Biosyst Eng 37:2325–2332. https://doi.org/10.1007/s00449-014-1210-9
Rahimnejad M, Adhami A, Darvari S, Zirepour A, Oh SE (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alex Eng J 54:745–756. https://doi.org/10.1016/j.aej.2015.03.031
Rajeswari S, Vidhya S, Navanietha Krishnaraj R, Saravanan P, Sundarapandiyan S, Maruthamuthu S, Ponmariappan S, Vijayan M (2016) Utilization of soak liquor in microbial fuel cell. Fuel 181:148–156. https://doi.org/10.1016/j.fuel.2016.04.121
Sawasdee V, Pisutpaisal N (2016) Simultaneous pollution treatment and electricity generation of tannery wastewater in air-cathode single chamber MFC. Int J Hydrog Energy 41:15632–15637. https://doi.org/10.1016/j.ijhydene.2016.04.179
Sevda S, Sreekrishnan TR (2012) Effect of salt concentration and mediators in salt bridge microbial fuel cell for electricity generation from synthetic wastewater. J Environ Sci Health A Tox Hazard Subst Environ Eng 47:878–886
Slate AJ, Whitehead KA, Brownson DAC, Banks CE (2019) Microbial fuel cells: an overview of current technology. Renew Sustain Energy Rev 101:60–81. https://doi.org/10.1016/j.rser.2018.09.044
Venkata Mohan S, Mohanakrishna G, Reddy BP, Saravanan R, Sarma PN (2008) Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (MFC) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment. Biochem Eng J 39:121–130. https://doi.org/10.1016/j.bej.2007.08.023
Venu D, Gandhimathi R, Nidheesh PV, Ramesh ST (2016) Effect of solution pH on leachate treatment mechanism of peroxicoagulation process. J Hazard Toxic Radioact Waste 20:4–7. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000315
Wang YP, Liu XW, Li WW, Li F, Wang YK, Sheng GP, Zeng RJ, Yu HQ (2012) A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Appl Energy 98:230–235. https://doi.org/10.1016/j.apenergy.2012.03.029
Yadav AK, Dash P, Mohanty A, Abbassi R, Mishra BK (2012) Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol Eng 47:126–131. https://doi.org/10.1016/j.ecoleng.2012.06.029
You S, Zhao Q, Zhang J, Jiang J, Zhao S (2006) A microbial fuel cell using permanganate as the cathodic electron acceptor. J Power Sources 162:1409–1415. https://doi.org/10.1016/j.jpowsour.2006.07.063
Zhang B, Zhao H, Zhou S, Shi C, Wang C, Ni J (2009) A novel UASB–MFC–BAF integrated system for high strength molasses wastewater treatment and bioelectricity generation. Bioresour Technol 100:5687–5693. https://doi.org/10.1016/j.biortech.2009.06.045
Zhang X, Zhu F, Chen L, Zhao Q, Tao G (2013) Removal of ammonia nitrogen from wastewater using an aerobic cathode microbial fuel cell. Bioresour Technol 146:161–168. https://doi.org/10.1016/j.biortech.2013.07.024
Zhu X, Logan BE (2013) Using single-chamber microbial fuel cells as renewable power sources of electro-Fenton reactors for organic pollutant treatment. J Hazard Mater 252–253:198–203. https://doi.org/10.1016/j.jhazmat.2013.02.051
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jayashree, S., Ramesh, S.T., Lavanya, A. et al. Wastewater treatment by microbial fuel cell coupled with peroxicoagulation process. Clean Techn Environ Policy 21, 2033–2045 (2019). https://doi.org/10.1007/s10098-019-01759-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-019-01759-0