Abstract
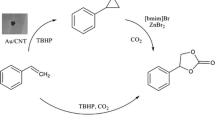
Development of environmentally benign green processes for utilization of carbon dioxide to synthesize value-added compounds using heterogeneous catalysis is desirable. One such proposed process is the cycloaddition of styrene oxide with carbon dioxide to synthesize styrene carbonate, synthesis of which has been well studied using various homogenous catalysts. The aim of the current work was to develop an active, selective and reusable heterogeneous catalyst for the cycloaddition of carbon dioxide to styrene oxide. Various solid catalysts such as 0.1% Li/MgO, calcined hydrotalcite (CHT), La–Zr mixed oxide (1:3 La/Zr), 0.1% La/MgO and ZrO2 were employed for cycloaddition of carbon dioxide to styrene oxide. Carboxylation of styrene oxide with carbon dioxide is enhanced by catalysts having both strong basic sites and weak acidic sites. Lanthanum–zirconia mixed oxide was prepared by combustion method using different compositions and evaluated for this reaction. La/Zr ratio of 1:3 was found to be the best among all studied and gave 90% conversion of styrene oxide with 100% selectivity of styrene carbonate at moderate temperature and pressure conditions in 3 h. The catalyst was characterized by various techniques to understand its textural properties. The catalyst was reused over three cycles without any loss of activity. The optimum reaction conditions were obtained by studying all process parameters in a laboratory batch stirred tank reactor (speed of agitation-1000 rpm, catalyst loading-2 × 10−2 g/cm3, 0.01 mol styrene oxide, DMF as solvent (total volume 30 mL), CO2 pressure-2.5 MPa, 130 °C). A detailed mathematical model was developed using Langmuir–Hinshelwood-Hougen–Watson mechanism in the absence of any transport resistances. The activation energy was calculated as 19.1 kcal/mol. The process is green and clean.















Similar content being viewed by others
Change history
20 March 2018
This paper is part of the special section Catalysis for sustainable development, but inadvertently
Abbreviations
- SO:
-
Styrene oxide
- La–Zr:
-
Lanthanum–zirconia mixed oxide
- P :
-
Reactant species, styrene oxide
- Q :
-
Reactant species, CO2
- A i :
-
Acidic site
- B i :
-
Basic sites
- PA 1 :
-
Chemisorbed P on acidic site A 1
- QB 2 :
-
Chemisorbed Q on basic site B 2
- SA 1 :
-
Chemisorbed styrene carbonate; S Styrene carbonate
- C P :
-
Concentration of P (mol/cm3)
- C Q :
-
Concentration of Q (mol/cm3)
- C S :
-
Concentration of S (mol/cm3)
- C PA1 :
-
Concentration of P at solid (catalyst) surface at site A1 (mol/g-cat)
- C QB2 :
-
Concentration of Q at solid (catalyst) surface at site B2 (mol/g-cat)
- C SA1 :
-
Concentration of S at solid (catalyst) surface at site A1 (mol/g-cat)
- C B2 :
-
Concentration of vacant sites (mol/g-cat)
- C t :
-
Total concentration of the sites (mol/g-cat)
- C t1 :
-
Total concentration of acidic sites (mol/g-cat)
- C t2 :
-
Total concentration of basic sites (mol/g-cat)
- K P , K Q :
-
Adsorption equilibrium constants for P and Q (g-cat/mol)
- K S :
-
Adsorption equilibrium constants for S (g-cat/mol)
- k 1 :
-
Forward reaction rate constant for surface reaction (cm6g-cat−1mol−1min−1)
- k 1′ :
-
Backward reaction rate constant for surface reaction (cm6g-cat−1mol−1min−1)
- w :
-
Catalyst loading (g/cm3)
- X P :
-
Fractional conversion of P
References
Adachi H (1991) Introduction to quantum material chemistry. Sankyo Inc, Tokyo, pp 123–132 (in Japanese)
Beattie C, North M, Villuendas P, Young C (2013) Influence of temperature and pressure on cyclic carbonate synthesis catalyzed by bimetallic aluminum complexes and application to overall syn-bis-hydroxylation of alkenes. J Org Chem 78:419–426
Chuah GK, Jaenicke S (1997) The preparation of high surface area zirconia—influence of precipitating agent and digestion. Appl Catal A Gen 163:261–273
Di Monte R, Kašpar J (2005) Nanostructured CeO2–ZrO2 mixed oxides. J Mater Chem 15:633–648
Ganduglia-Pirovano MV, Hofmann A, Sauer J (2007) Oxygen vacancies in transition metal and rare earth oxides: current state of understanding and remaining challenges. Surf Sci Rep 62:219–270
Horiuchi T, Hidaka H, Fukui T et al (1998) Effect of added basic metal oxides on CO2 adsorption on alumina at elevated temperatures. Appl Catal A Gen 167:195–202
Hu MZ, Hunt RD, Payzant EA, Hubbard CR (1999) Nanocrystallization and phase transformation in monodispersed ultrafine zirconia particles from various homogeneous precipitation methods. J Am Ceram Soc 82:2313–2320
Kawanami H, Ikushima Y (2000) Chemical fixation of carbon dioxide to styrene carbonate under supercritical conditions with DMF in the absence of any additional catalysts. Chem Commun (21):2089–2090
Kim YJ, Varma RS (2005) Tetrahaloindate (III)-based ionic liquids in the coupling reaction of carbon dioxide and epoxides to generate cyclic carbonates: H-bonding and mechanistic studies. J Org Chem 70:7882–7891
Li F, Xiao L, Xia C, Hu B (2004) Chemical fixation of CO2 with highly efficient ZnCl2/[BMIm] Br catalyst system. Tetrahedron Lett 45:8307–8310
Li W, Huang H, Li H et al (2008) Facile synthesis of pure monoclinic and tetragonal zirconia nanoparticles and their phase effects on the behavior of supported molybdena catalysts for methanol-selective oxidation. Langmuir 24:8358–8366
Liu Z, Ji W, Dong L, Chen Y (1998) Effect of supported Na+ Ions on the texture properties of ZrO2. J Solid State Chem 138:41–46
Lu X-B, Zhang Y-J, Liang B et al (2004) Chemical fixation of carbon dioxide to cyclic carbonates under extremely mild conditions with highly active bifunctional catalysts. J Mol Catal A Chem 210:31–34
Meléndez J, North M, Villuendas P, Young C (2011) One-component bimetallic aluminium (salen)-based catalysts for cyclic carbonate synthesis and their immobilization. Dalton Trans 40:3885–3902
Mercera PDL, Van Ommen JG, Doesburg EBM et al (1991) Zirconia as a support for catalysts Influence of additives on the thermal stability of the porous texture of monoclinic zirconia. Appl Catal 71:363–391
Paddock RL, Nguyen ST (2001) Chemical CO2 fixation: Cr(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J Am Chem Soc 123:11498–11499
Piticescu RR, Monty C, Taloi D et al (2001) Hydrothermal synthesis of zirconia nanomaterials. J Eur Ceram Soc 21:2057–2060
Prastomo N, Muto H, Sakai M, Matsuda A (2010) Formation and stabilization of tetragonal phase in sol–gel derived ZrO2 treated with base-hot-water. Mater Sci Eng B 173:99–104
Qiao K, Ono F, Bao Q et al (2009) Efficient synthesis of styrene carbonate from CO2 and styrene oxide using zinc catalysts immobilized on soluble imidazolium–styrene copolymers. J Mol Catal A Chem 303:30–34
Shukla S, Seal S, Vij R et al (2002) Effect of nanocrystallite morphology on the metastable tetragonal phase stabilization in zirconia. Nano Lett 2:989–993
Song J, Zhang Z, Hu S et al (2009) MOF-5/n-Bu4NBr: an efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2 under mild conditions. Green Chem 11:1031–1036
Srivastava R, Srinivas D, Ratnasamy P (2003) Synthesis of polycarbonate precursors over titanosilicate molecular sieves. Catal Lett 91:133–139
Sun J, Fujita S, Zhao F, Arai M (2004) Synthesis of styrene carbonate from styrene oxide and carbon dioxide in the presence of zinc bromide and ionic liquid under mild conditions. Green Chem 6:613–616
Sun J, Fujita S-I, Zhao F, Arai M (2005) A highly efficient catalyst system of ZnBr2/n-Bu4NI for the synthesis of styrene carbonate from styrene oxide and supercritical carbon dioxide. Appl Catal A Gen 287:221–226
Tambe PR, Yadav GD (2017) Selective carbonylation of o-phenylene diamine using carbon dioxide as feedstock for synthesis of 1, 3-dihydro-benzimidazol-2-one over La–Zr mixed oxide. J Clean Prod 166:285–298. https://doi.org/10.1016/j.jclepro.2017.08.025
Thangadurai P, Bose AC, Ramasamy S (2005) Phase stabilization and structural studies of nanocrystalline La2O3–ZrO2. J Mater Sci 40:3963–3968
Tiwari MS, Yadav GD (2015) Kinetics of Friedel-Crafts benzoylation of veratrole with benzoic anhydride using Cs2.5H0.5PW12O40/K-10 solid acid catalyst. Chem Eng J 266:64–73
Tiwari MS, Yadav GD (2016) Novel aluminium exchanged dodecatungstophosphoric acid supported on K-10 clay as catalyst: benzoylation of diphenyloxide with benzoic anhydride. RSC Adv 6:49091–49100
Tiwari MS, Gawade AB, Yadav GD (2017) Magnetically separable sulfated zirconia as highly active acidic catalysts for selective synthesis of ethyl levulinate from furfuryl alcohol. Green Chem 19:963–976
Ulusoy M, Cetinkaya E, Cetinkaya B (2009) Conversion of carbon dioxide to cyclic carbonates using diimine Ru (II) complexes as catalysts. Appl Organomet Chem 23:68–74
Wang H, Li G, Xue Y, Li L (2007) Hydrated surface structure and its impacts on the stabilization of t-ZrO2. J Solid State Chem 180:2790–2797
Wang S, Xie H, Lin Y et al (2016) High thermal stability of La2O3-and CeO2-stabilized tetragonal ZrO2. Inorg Chem 55:2413–2420
Wong W, Chan P, Zhou Z et al (2008) A robust ionic liquid as reaction medium and efficient organocatalyst for carbon dioxide fixation. ChemSusChem 1:67–70
Xie Y, Zhang Z, Jiang T et al (2007) CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew Chem 119:7393–7396
Yamaguchi K, Ebitani K, Yoshida T et al (1999) Mg–Al mixed oxides as highly active acid-base catalysts for cycloaddition of carbon dioxide to epoxides. J Am Chem Soc 121:4526–4527
Yang Z, He L, Miao C, Chanfreau S (2010) Lewis basic ionic liquids-catalyzed conversion of carbon dioxide to cyclic carbonates. Adv Synth Catal 352:2233–2240
Yano T, Matsui H, Koike T et al (1997) Magnesium oxide-catalysed reaction of carbon dioxide with an epoxide with retention of stereochemistry. Chem Commun (12):1129–1130
Zhao T, Han Y, Sun Y (1999) Cycloaddition between propylene oxide and CO2 over metal oxide supported KI. Phys Chem Chem Phys 1:3047–3051
Zhu M, Srinivas D, Bhogeswararao S et al (2013) Catalytic activity of ZIF-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catal Commun 32:36–40
Acknowledgements
This work was done under the collaborative project “Sustainable Catalytic Syntheses of Chemicals using Carbon Dioxide as Feedstock (GreenCatCO2)” supported by the Department of Science and Technology, Government of India (DST-GOI) and the Academy of Finland. GDY acknowledges support from R.T. Mody Distinguished Professor Endowment and J.C. Bose National Fellowship from DST-GOI. Pooja Tambe acknowledges the Department of Science and Technology for awarding the Junior Research Fellowship under Indo-Finnish Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10098-018-1517-3.
Rights and permissions
About this article
Cite this article
Tambe, P.R., Yadav, G.D. Heterogeneous cycloaddition of styrene oxide with carbon dioxide for synthesis of styrene carbonate using reusable lanthanum–zirconium mixed oxide as catalyst. Clean Techn Environ Policy 20, 345–356 (2018). https://doi.org/10.1007/s10098-017-1475-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-017-1475-1