Abstract
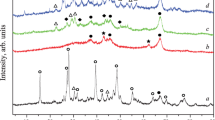
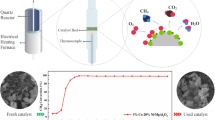
A series of Ru/Co/La/Al2O3 supported on alumina (Al2O3) were prepared to investigate its catalytic study which aimed the performance of the catalyst. The potential catalysts were subjected to calcination at various temperatures in order to investigate the physicochemical properties of the oxides affected by the parameter. The samples were then characterized by X-ray diffraction (XRD), field emission scanning electron microscopy–energy dispersive X-ray (FESEM–EDX), and Brunauer, Emmett, and Teller analyses. It was found that phase evolutions took place after subjected to calcination at various temperatures. However, the calcination temperatures did not significantly affect the morphology, surface area, and particle size of the catalysts. The FESEM analysis revealed that fresh nanosized Ru/Co/La(5:35:60)/Al2O3 catalyst was obtained with agglomeration and not homogenously dispersed. In the reduction process, smaller particles were found to be more difficult to be reduced than the larger particles. From the EDX, the expected elements were observed, and aluminum (Al) was found to be the most dominant. Besides that, the XRD analysis performed on Ru/Co/La(5:35:60)/Al2O3 catalyst at calcination temperatures of 900, 1,000, and 1,100 °C showed very low degree of crystallinity and were dominated by Al2O3 as the support.









Similar content being viewed by others
References
Ahmed TY, Ahmad MM, Lam HL, Yusup S (2013) Hydrogen from renewable palm kernel shell via enhanced gasification with low carbon dioxide emission. Clean Technol Environ Policy 15:513–523. doi:10.1007/s10098-013-0606-6
Andersson JM (2005) Controlling the formation and stability of alumina phases. Ph.D. Thesis, Linkopings Universitet, Sweden, pp 26–33
Bartholomew CH (2011) Carbon deposition in stream reforming and methanation. Appl Catal A 212:17–60
Cao Q, Yu Q, Des Connell W, Yu G (2013) Titania/carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technnol Environ Policy 15:871–880. doi:10.1007/s10098-013-6581-y
Hussain A, Saiyudi NK, Majid Z (2008) Introduction to surface and colloid chemistry. Master Thesis, Johor: Universiti Teknologi Malaysia, Malaysia, pp 49–54
Kok E, Scott J, Cant N, Trimm D (2011) The impact of ruthenium, lanthanum and activation conditions in the methanation activity of alumina-supported cobalt catalysts. Catal Today 164:297–301
Li Y (2014) Rare earth alumina particulate manufacturing method and application. US 8,629,077B2. Washington DC: United State Patent
Oh SW, Bang HY, Bae YC, Sun YK (2007) Effect of calcinations temperature on morphology, crystallinity and electrochemical properties of nanocrystalline metal oxides (Co3O4, CuO and NiO) prepared via ultrasonic spray pyrolysis. J Power Sources 173:502–509
Opanasenko M, Amarajothi Dhakshinamoorthy, Cejka J, Garcia H (2013) Deactivation pathways of the catalytic activity of metal-organic frameworks in condensation reactions. Chem Cat Chem 5(60):1553–1561
Palero MLY, Abella LC, Monroy TG (2012) Optimization of process parameters of methane decomposition in a fluidized bed reactor using Ni–Cu/Al2O3 catalysts. Int J Chem Eng Appl 3:157
Palma V, Castaldo F, Ciambelli P, Laqueriello G (2012) Hydrogen production through catalytic low-temperature bio-ethanol steam reforming. Clean Technol Environ Policy 14:973–987. doi:10.1007/s10098-012-0472-7
Panagiotopoulou P, Kondarides DI, Verykios XE (2009) Selective methanation of CO over supported Ru catalysts. Appl Catal B 88(3–4):470–478
Wan Abu Bakar WA, Othman MY, Ali R, Ching KY, Toemen S (2009a) The investigation of active sites on nickel oxide based catalysts towards the in-situ reaction of methanation and desulfurization. Mod Appl Sci 3:35–41
Wan Abu Bakar WA, Othman MY, Ali R, Ching KY (2009b) Nickel oxide based supported catalysts for the in-situ reactions of methanation and desulfurization in the removal of sour gases from simulated natural gas. Catal Lett 128:127–136
Wan Azelee WAB, Rusmidah A, Abdul Aziz AK, Salmiah Jamal MR, Nurul SM (2012) Catalytic methanation reaction over alumina supported cobalt oxide doped noble metal oxides for the purification of simulated natural gas. J Fuel Chem Technol 40(7):822–830
Yamamoto T, Hatsui T, Matsuyama T, Tanaka T, Funabiki T (2003) Structure and acid–base properties of La/Al2O3-role of La addition to enhance thermal stability of gamma Al2O3. Chem Mater 15:4830–4840. doi:10.1021/cm034732c
Yousef SH, Najjar (2008) Modern and appropriate technologies for reducing of gaseous pollutants and their effects on the environments. Clean Technol Environ Policy 10:269–278. doi:10.1007/s10098-007-0111-x
Zhao L, Ma J, Sun Z, Zhai X (2008) Catalytic ozonation for the degradation of nitrobenzene in aqueous solution by ceramic honeycomb-supported manganese. Appl Catal B 83:256–264
Acknowledgement
We thank to the Universiti Teknologi Malaysia, Ministry of Higher Education (MOHE), Malaysia for the financial support given under the GUP, Vot 04H97 and Ministry of Science, Technology and Innovation (MOSTI) for My Brain 15 (My Ph.D.) scholarship to Salmiah Jamal Mat Rosid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mat Rosid, S.J., Wan Abu Bakar, W.A. & Ali, R. Physicochemical study of supported cobalt–lanthanum oxide-based catalysts for Co2/H2 methanation reaction. Clean Techn Environ Policy 17, 257–264 (2015). https://doi.org/10.1007/s10098-014-0766-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0766-z