Abstract
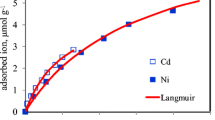
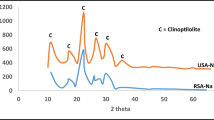
The competitive adsorption behavior of cadmium (Cd2+), copper (Cu2+), nickel (Ni2+), and lead (Pb2+) ions using Iranian natural zeolite has been studied in order to determine its applicability in treating industrial wastewater. Tests to determine both the rate of adsorption and the uptake at equilibrium were performed under batch conditions from single- and multi-component solutions. The optimum conditions for the treatment process were investigated by observing the influence of pH levels, the presence of competing ions, varying the mass of zeolite and different contact time. Adsorption kinetics of the zeolite followed first-order kinetics, showing about 100 % of Pb2+ removal within 40 min and reaching an equilibrium state within 24 h for Cd2+, Cu2+, and Ni2+. The results indicated that removal of metals from single- and multi-component solutions is best described by a Freundlich isotherm, in which the distribution coefficient was in the following order: Pb2+ > Cu2+ > Cd2+ > Ni2+. In the multi-component solutions, metals exhibit competitive adsorption on the zeolite. The adsorption is reduced to 90, 53, 30, and 22 % of single component of Cu2+, Ni2+, Cd2+, and Pb2+, respectively. However, the total adsorption was higher than single component. Finally, soil solution saturation indices and speciation of metals was assessed using Visual MINTEQ 2.6 software, and probability of precipitation of minerals supported by scanning electron microscopy. The research indicates that Cd2+ and Ni2+ retention by zeolite can be viewed as the result of ion exchange reaction, but Pb2+ and Cu2+ retention is both due to ion exchange and precipitation. These results show that Iranian natural zeolite particularly effective in removing cationic heavy metal species from industrial wastewater.









Similar content being viewed by others
References
Álvarez-Ayuso E, García-Sánchez A, Querol X (2003) Purification of metal electroplating waste waters using zeolites. Water Res 37:4855–4862
Amarasinghe BMWPK, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
An HK, Park BY, Kim DS (2001) Crab shell for removal of heavy metals from aqueous solution. Water Res 35:3551–3556
Babel S, Kurniawan TA (2003) Low-cost adsorbent for heavy metals uptake from contaminated water: a review. J Hazard Mater B97:219–243
Biskup B, Subotic B (2004) Kinetic analysis of the exchange processes between sodium ions from zeolite A and cadmium, copper and nickel ions from solutions. Sep Purif Technol 37:17–31
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507
Brown G Jr, Foster A, Ostergren JD (1999) Mineral surfaces and bioavailability of heavy metals: A molecular-scale perspective. Proc. Natl. Acad. Sci. 96:3388–3395
Brümmer GW, Gerth J, Tiller KG (1988) Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite: I. Adsorption and diffusion of metals. J Soil Sci 39:37–52
Cabrera C, Gabald′on C, Marzal P (2005) Sorption characteristics of heavy metal ions by a natural zeolite. J Chem Technol Biotechnol 80:477–481
Cincotti A, Mameli A, Locci AM, Orru R, Cao G (2006) Heavy metals uptake by Sardinian natural zeolites: experiment and modeling. Ind Eng Chem Res 45:1074–1084
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Elzinga EJ, Rouff AA, Reeder RJ (2006) The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: an X-ray absorption spectroscopy study. Geochim Cosmochim Acta 70:2715–2725
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Ersoy B, Çelik MS (2002) Electrokinetic properties of clinoptilolite with mono- and multivalent electrolytes. Micropor Mesopor Mater 55:305–312
Flores V, Cabassud C (1999) A hybrid membrane process for Cu(II) removal from industrial wastewater, comparison with a conventional process system. Desalination 126:101–108
Gustafsson JP (2006) VISUAL MINTEQ. http://www.lwr.kth.se./English/OurSoftware/vMINTEQ/, Stockholm, Sweden. Accessed Dec 2006
Haidouti C (1997) Inactivation of mercury in contaminated soils using natural zeolites. Sci Total Environ 208:105–109
Jelas Haron Md, Siang PO, Kassim A (2008) Sorption of arsenate by stannum(iv)-exchanged zeolite p. Malays J Anal Sci 12(2):310–321
Ho Y, Porter J, McKay G (2002) Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 141:1–33
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2003) Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake. J Colloid Interface Sci 261:49–54
Langella A, Pansini M, Cappelletti P, De Gennaro B, De’ Gennaro M, Colella C (2000) NH4 +, Cu2+, Zn2+, Cd2+ and Pb2+ exchange for Na+ in a sedimentary clinoptilolite, North Sardinia, Italy. Micropor Mesopor Mater 37:337–343
Leppert D (1990) Heavy metal sorption with clinoptilolite zeolite alternatives for treating contaminated soil and water. Min Eng 42:604–608
Lin SH, Juang RS (2002) Heavy metal removal from water by sorption using surfactant modified montmorillonite. J Hazard Mater B 92:315–326
Lu A, Zhang S, Sham X-Q (2005) Time effect on the fractionation of heavy metals in soils. Geoderma 125:225–234
Malliou E, Malamis M, Sakellarides PO (1992) Lead and cadmium removal by ion exchange. Water Sci Technol 25:133–138
Morera MT, Echeverria JC, Mazkiaran C, Garrido JJ (2001) Isotherm and sequential extraction procedures for evaluating sorption and distribution of heavy metals in soils. Environ Pollut 113:135–144
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92:42–48
Mozgawa W (2004) The influence of some heavy metals cations on the FTIR spectra of zeolites. J Mol Struct 555:299–304
Myroslav S, Boguslaw B, Artur TP, Jacek N (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+ and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304:21–28
Nomanbhay SM, Palanisamy K (2005) Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron J Biotechnol 8:43–53
Noroozifar M, Motlagh KM, Fard AP (2009) Cyanide uptake from wastewater by modified natrolite zeolite–iron oxyhydroxide system: application of isothermand kinetic models. J Hazard Mater 166:1060–1066
Ok YS, Yang JE, Zhang Y-S, Kim SJ, Chung DY (2007) Heavy metal adsorption by a formulated zeolite–Portland cement mixture. J Hazard Mater 147:91–96
Ören AH, Kaya A (2006) Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J Hazard Mater 131:59–65
Oter O, Akcay H (2007) Use of natural clinoptilolite to improve water quality: sorption and selectivity studies of lead(II), copper(II), zinc(II), and nickel(II), Water Environ. Res. 79:329–335
Ouki SK, Kavanagh M (1997) Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Manag Res 15:383–394
Ouki SK, Kavannagh M (1999) Treatment of metal-contaminated waste waters by use of natural zeolites. Water Sci Technol 39:115–122
Ouki SK, Cheeseman CR, Perry R (1993) Effects of conditioning and treatment of chabazite and clinoptilolite prior to lead and cadmium removal. Environ Sci Technol 27:1108–1116
Panayotova M (2001) Kinetics and thermodynamics of removal of nickel ions from wastewater by use of natural and modified zeolite. Fresenius Environ Bull 10:267–272
Panayotova M, Velikov B (2002) Kinetics of heavy metal ions removal by use of natural zeolite. J Environ Sci Health A 37:139–147
Panayotowa MI (2003) Kinetics and thermodynamic of copper ions removal from wastewater by use of zeolite. Waste Manag 23:135–143
Pedersen AJ (2002) Evaluation of assisting agents for electrodialytic removal of Cd, Pb, Zn, Cu and Cr from MSWI fly ash. J Hazard Mater B95:185–198
Peric J, Trgo M, Medvidivic NV (2004) Removal of zinc, copper and lead by natural zeolite—a comparison of adsorption isotherms. Water Res 38:1893–1899
Poon CPC (1986) Removal of cadmium from waste waters. In: Mislin H, Ravera O (eds) Cadmium in the environment. Birkha User, Basel, pp 46–55
Shibata W, Seff K (1997) Pb2+ exchange isotherms for zeolite Na-X at pH 5, 6, and 7. Zeolites 19:87–89
Sprynskyy M, Buszewski B, Terzyk AP, Namiesnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304:21–28
Trgo M, Perić J (2003) Interaction of the zeolitic tuff with Zn-containing simulated pollutant solutions. J Colloid Interface Sci 260:166–175
Trgo M, Peric J, Medvidovic N (2006) Investigations of different kinetic models for zinc ions uptake by a natural zeolitic tuff. J Environ Manag 79:298–304
Turkman A, Aslan S, Ege I (2004) Treatment of metal containing wastewaters by natural zeolites. Fresenius Environ Bull 13:574–580
Vidal M, Santos MJ, Abrao T, Rodriguez J, Rigol A (2009) Modeling competitive metal sorption in a mineral soil. Geoderma 149:189–198
Wang Sh, Terdkiatburana T, Tadé MO (2008) Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems. Sep Purif Technol 62:64–70
Weng LP, Temminghoff EJM, van Riemsdijk WH (2001) Contribution of individual sorbents to the control of heavy metal activity in sandy soil. Environ Sci Technol 35:4436–4443
Zamzow MJ, Eichbaum BR, Sandgren KR, Shanks DE (1990) Removal of heavy metals and other cations from waste water using Zeolites. Sep Sci Technol 25:1555–1569
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merrikhpour, H., Jalali, M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite. Clean Techn Environ Policy 15, 303–316 (2013). https://doi.org/10.1007/s10098-012-0522-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-012-0522-1