Abstract
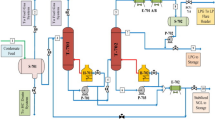
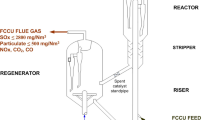
In this case study, the process modifications and improvement to the existing process at the Emirates Gold refinery (in order to meet the United Arab Emirates (UAE) nitrogen oxides air emission regulations) is presented. In the past, Emirates Gold refinery used a single small scrubber to treat waste gases. In order to treat the waste gas efficiently, it was found that a cooled oxidation reactor (oxidizer) before the existing scrubber, as well as a second scrubber is needed. The waste gas is mixed with air at a fixed ratio before entering the oxidizer which is designed to obtain the optimum degree of NO oxidation (about 50%).To keep the oxidation reactions in the desirable direction the temperature should be kept between 15 and 20°C There for an internal cooler was required. The gas mixture from the Oxidizer enters the first scrubber (existing) where most of the NO x , mainly as N2O3, are absorbed by a NaOH solution (15–20%). The remaining NO x , mainly as N2O3 is absorbed in the second scrubber by a NaOH solution (8–10%). The mass transfer area of the packing in the two scrubbers, the solution circulation rate, and the cooling duty were designed to reach the desired degree of absorption of N2O3 and NO2. This ensures that the recommended NO x residual value of 500 mg/m3 (250 ppm) is reached. All reactions occur simultaneously was calculated using EQ4WIN software. The data obtained for different temperatures was processed with Stat View, SuperPro Designer simulation and Aspen HYSYS simulation.



Similar content being viewed by others
References
Barnes JM, Apel WA, Barrett KB (1995) Removal of nitrogen oxides from gas streams using biofiltration. J Hazard Mater 41(2–3):315–326
Chien T, Chu H (2000) Removal of SO2 and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution. J Hazard Mater B80:43–57
Chironna R, Altshuler B (2009) Chemical aspects of NO x scrubbing. Environmental Center. http://www.environmental-center.com/articles/article1011/article1011.htm. Accessed 23 March 2010
Coates TJ (1987) Absorption from air streams using aqueous solutions. AIChE Summer National Meeting, Technical Paper 9C
du Plessis CA, Kinney KA, Schroeder ED, Chang DPY, Scow KM (1998) Denitrification and nitric oxide reduction in an aerobic toluene-treating biofilter. Biotechnol Bioeng 58(4):408–415
Dubai Municipality (2008) Air quality monitoring in Dubai. Government of Dubai. https://portal.dm.gov.ae/AirQuality/AirqualityStandards.htm. Accessed 12 Apr 2010
Economidis N, Coil R, Smirniotis P (1998) Catalytic performance of Al2O3/SiO2/TiO2 loaded with V2O5 for the selective catalytic reduction of NO x with ammonia. Catal Today 40(1):27–37
Flanagan WP, Apel WA, Barnes JM, Lee BD (2002) Development of gas phase bioreactors for the removal of nitrogen oxides from synthetic flue gas streams. Fuel 81:1953–1961
Günther L (2010) NO x separation from drawn off air: a method of recovery of a high-quality substance from drawn off air without any waste formation. DGE- Nox-Entfernung aus Abluft. http://www.dge-wittenberg.de/english/produkte/waescherprogramm/nox.html. Accessed 20 April 2010
Heidenreich S, Nacken M, Hackel M, Schaub G (2008) Catalytic filter elements for combined particle separation and nitrogen oxides removal from gas streams. Powder Technol 180:86–90
Lietti L (1996) Reactivity of V2O5&z.sbnd;WO3TiO2 de-NO x catalysts by transient methods. Appl Catal B 10(4):281–297
Massucci M, Clegg SL, Brimblecombe P (1999) Equilibrium partial pressures, thermodynamic properties of aqueous and solid phases, and Cl2 production from aqueous HCl and HNO3 and their mixtures. J Phys Chem A 103(21):4209–4226
Patwardhan JA, Joshi JB (2004) Unified model for NO x absorption in aqueous alkaline and dilute acidic solutions. AIChE J 49(11):2728–2748
Qian Z et al (2010) Seasonal pattern of the acute mortality effects of air pollution. J Air Waste Manag Assoc 60:481–488
Radojevic M (1998) Reduction of nitrogen oxides in flue gases. Environ Pollut 102(1):685–689
Thomas D, Vanderschuren J (1996) The absorption–oxidation of NO x with hydrogen peroxide for the treatment of tail gases. Chem Eng Sci 51(11):2649–2654
Thomas D, Vanderschuren J (2000) Nitrogen oxides scrubbing with alkaline solutions. Chem Eng Technol 23(5):449–455
Wallin M, Karlsson CJ, Skoglundh M, Anders P (2003) Selective catalytic reduction of NO x with NH3 over H-ZSM-5: Influence of transient ammonia supply. J Catal 218:354–364
Acknowledgments
The authors are grateful to Mr. Mohamed Shekarchy, Director of Emirates Gold DMCC, and Engineer Faisal Dawood for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aidan, A., Alnaizy, R., Nenov, V. et al. Process design of waste gas treatment from Emirates Gold Refinery. Clean Techn Environ Policy 13, 447–457 (2011). https://doi.org/10.1007/s10098-010-0323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-010-0323-3