Abstract
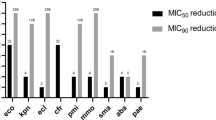
Sitafloxacin is one of the newer generation fluoroquinolones. Considering the ever-changing antimicrobial resistance, it is necessary to monitor the activities of sitafloxacin against recent pathogenic isolates. Therefore, we determined the minimum inhibitory concentrations (MICs) of sitafloxacin and comparators by broth microdilution or agar dilution method against 1101 clinical isolates collected from 2017 to 2019 in 31 hospitals across China. Sitafloxacin was highly active against gram-positive isolates evidenced by the MICs required to inhibit the growth of 50%/90% isolates (MIC50/90): ≤ 0.03/0.25, ≤ 0.03/0.125, ≤ 0.03/2, 0.125/0.25, 0.25/2, and 0.125/0.125 mg/L for methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-susceptible coagulase-negative Staphylococcus (MSCNS), methicillin-resistant S. aureus (MRSA), methicillin-resistant CNS, Enterococcus faecalis, and Streptococcus pneumoniae, respectively. Sitafloxacin inhibited 82.8% of the MRSA strains and 97.5% of MRCNS strains. Sitafloxacin was also potent against ciprofloxacin-susceptible Escherichia coli (MIC50/90: ≤ 0.03/0.06 mg/L) and Klebsiella pneumoniae (MIC50/90: ≤ 0.03/0.125 mg/L), non-ESBL-producing E. coli (MIC50/90: ≤ 0.03/1 mg/L) and K. pneumoniae (MIC50/90: ≤ 0.03/0.5 mg/L), Haemophilus influenzae (MIC50/90: ≤0.015/0.06 mg/L), Haemophilus parainfluenzae (MIC50/90: 0.125/0.5 mg/L), Moraxella catarrhalis (MIC50/90: ≤ 0.015/≤ 0.015 mg/L), Bacteroides fragilis (MIC50/90: 0.06/2 mg/L), Peptostreptococcus (MIC50/90: 0.125/4 mg/L), and Mycoplasma pneumoniae (≤ 0.03/≤ 0.03 mg/L). However, sitafloxacin was less active for Enterococcus faecium, ciprofloxacin-resistant and/or ESBL-producing E. coli, and K. pneumoniae strains. Sitafloxacin was superior or comparable to most of the comparators in activities against the abovementioned isolates, so sitafloxacin is still highly active against most of the clinical isolates in hospitals across China, proving its utility in treatment of the abovementioned susceptible strains.

Similar content being viewed by others
Data availability
Data can be provided upon request.
References
Asakura T, Suzuki S, Fukano H et al (2019) Sitafloxacin-containing regimen for the treatment of refractory Mycobacterium avium complex lung disease. Open Forum Infect Dis 6:ofz108
Manosuthi W, Wiboonchutikul S (2016) Treatment outcomes of oral sitafloxacin in acute complicated urinary tract infection and pyelonephritis. Springerplus 5:410
Keating GM (2011) Sitafloxacin: in bacterial infections. Drugs 71:731–744
Hamasuna R, Ohnishi M, Matsumoto M et al (2018) In Vitro activity of sitafloxacin and additional newer generation fluoroquinolones against ciprofloxacin-resistant Neisseria gonorrhoeae isolates. Microb Drug Resist 24:30–34
Tantisiriwat W, Linasmita P (2017) In vitro activity of sitafloxacin and other antibiotics against bacterial isolates from HRH Princess Maha Chakri Sirindhorn Medical Center, Srinakharinwirot University and Samitivej Sukhumvit Hospital. J Med Assoc Thail 100:469–478
Xu N, Wang G, Leng Y et al (2018) Sulbactam enhances the in vitro activity of sitafloxacin against extensively-drug resistant Acinetobacter baumannii. ExpTher Med 16:3485–3491
Clinical and Laboratory Standards Institute (2020) Performance standards for antimicrobial susceptibility testing; M100 30th. Clin Lab Stand Instit
CLSI (2018) M07, 11th Ed. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute, Wayne
CLSI (2011) Methods for antimicrobial susceptibility testing for human mycoplasmas; approved guideline (M43-A). Clinical and Laboratory Standards Institute, Wayne
Hu F, Guo Y, Yang Y et al (2019) Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis 38:2275–2281
Milatovic D, Schmitz FJ, Brisse S, Verhoef J, Fluit AC (2000) In vitro activities of sitafloxacin (DU-6859a) and six other fluoroquinolones against 8,796 clinical bacterial isolates. Antimicrob Agents Chemother 44:1102–1107
Schmitz FJ, Fluit AC, Milatovic D et al (2000) In vitro potency of moxifloxacin, clinafloxacin and sitafloxacin against 248 genetically defined clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 46:109–113
Amano A, Matsuzaki K, Kishi N et al (2013) In vitro activity of sitafloxacin against clinical isolates in 2012. Jpn J Antibiot 66:311–330 [Japanese]
Doern GV, Heilmann KP, Huynh HK et al (2001) Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob Agents Chemother 45:1721–1729
Snydman DR, Jacobus NV, McDermott LA et al (2002) In vitro activities of newer quinolones against Bacteroides group organisms [Errata in Antimicrob Agents Chemother 2003;47(2):831]. Antimicrob Agents Chemother 46:3276–3279
Tanigawara Y, Kaku M, Totsuka K, Tsuge H, Saito A (2013) Population pharmacokinetics and pharmacodynamics of sitafloxacin in patients with community-acquired respiratory tract infections. J Infect Chemother 19:858–866
Code availability
Not applicable.
Funding
This work was supported by National Mega-project for Innovative Drugs (2019ZX09721001-006-004), and CHINET Antimicrobial Surveillance Network (grant number WI207259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of Huashan Hospital, Fudan University (number: 2019-572).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, S., Yang, Y., Guo, Y. et al. Comparative activities of sitafloxacin against recent clinical isolates in hospitals across China. Eur J Clin Microbiol Infect Dis 40, 2271–2283 (2021). https://doi.org/10.1007/s10096-021-04278-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04278-3