Abstract
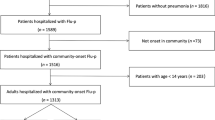
The aim of this study is to evaluate the impact of early (within 2 days after disease onset) neuraminidase inhibitor (NAI) administration on clinical outcomes in patients with laboratory-confirmed influenza B-related pneumonia (FluB-p). This was a multicenter study conducted from 1 January 2013 to 1 May 2019. Data of immunocompetent adult and adolescent FluB-p patients hospitalized at five different teaching hospitals in China were retrospectively collected, including demographic and clinical features as well as clinical and treatment outcomes. Univariate and multivariate logistic regression analyses were performed to assess the effects of early NAI administration on clinical outcomes in FluB-p patients. In total, 386 hospitalized patients with community-onset FluB-p were included in this study, of whom 39.6% (153/386) were treated with NAI early. After adjusting for the weighted propensity scores of treatment, systemic corticosteroid, and antibiotic uses, the results of multivariate logistic regression model indicated that early NAI treatment was associated with the decreased risks of invasive ventilation [odd ratio (OR) 0.325, 95% confidence interval (CI) 0.123–0.858; p = 0.023), admittance to intensive care unit (OR 0.425, 95% CI 0.204–0.882; p = 0.022), and 30-day mortality (OR 0.416, 95% CI 0.184–0.944, p = 0.036)] in FluB-p patients. In addition, the multivariate logistic regression analysis revealed that early NAI treatment (OR 0.306, 95% CI 0.063–0.618; p = 0.010) was an independent predictor for 30-day mortality in patients with FluB-p. Early NAI treatment was associated with better clinical outcomes in FluB-p patients, which supports the recommendations of its use in severe influenza illness.



Similar content being viewed by others
References
Reed C, Chaves SS, Daily Kirley P et al (2015) Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 10:e0118369. https://doi.org/10.1371/journal.pone.0118369
Thommes EW, Kruse M, Kohli M et al (2017) Review of seasonal influenza in Canada: burden of disease and the cost-effectiveness of quadrivalent inactivated influenza vaccines. Hum Vaccin Immunother 13:867–876. https://doi.org/10.1080/21645515.2016.1251537
Lee VJ, Ho ZJM, Goh EH et al (2018) Advances in measuring influenza burden of disease. Influenza Other Respir Viruses 12:3–9. https://doi.org/10.1111/irv.12533
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2095–2128. https://doi.org/10.1016/S0140-6736(12)61728-0
Ly S, Arashiro T, Ieng V et al (2017) Establishing seasonal and alert influenza thresholds in Cambodia using the WHO method: implications for effective utilization of influenza surveillance in the tropics and subtropics. Western Pac Surveill Response J 8:22–32. https://doi.org/10.5365/WPSAR.2017.8.1.002
Cowling BJ, Caini S, Chotpitayasunondh T et al (2017) Influenza in the Asia-Pacific region: findings and recommendations from the global influenza initiative. Vaccine 35:856–864. https://doi.org/10.1016/j.vaccine.2016.12.064
von Itzstein M, Wu WY, Kok GB et al (1993) Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 363:418–423. https://doi.org/10.1038/363418a0
He G, Massarella J, Ward P (1999) Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet 37:471–484. https://doi.org/10.2165/00003088-199937060-00003
Treanor JJ, Hayden FG, Vrooman PS et al (2000) Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA 283:1016–1024. https://doi.org/10.1001/jama.283.8.1016
South East Asia Infectious Disease Clinical Research Network (2013) Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ 346:f3039. https://doi.org/10.1136/bmj.f3039
Hanshaoworakul W, Simmerman JM, Narueponjirakul U et al (2009) Severe human influenza infections in Thailand: oseltamivir treatment and risk factors for fatal outcome. PLoS One 4:e6051. https://doi.org/10.1371/journal.pone.0006051
McGeer A, Green KA, Plevneshi A et al (2007) Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 45:1568–1575. https://doi.org/10.1086/523584
Barr IG, Jelley LL (2012) The coming era of quadrivalent human influenza vaccines: who will benefit? Drugs 72:2177–2185. https://doi.org/10.2165/11641110-000000000-00000
Caini S, Kusznierz G, Garate VV et al (2019) The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS One 14:e0222381. https://doi.org/10.1371/journal.pone.0222381
Fu X, Zhou Y, Wu J et al (2019) Clinical characteristics and outcomes during a severe influenza season in China during 2017-2018. BMC Infect Dis 19:668. https://doi.org/10.1186/s12879-019-4181-2
Shi W, Ke C, Fang S et al (2019) Co-circulation and persistence of multiple A/H3N2 influenza variants in China. Emerg Microbes Infect 8:1157–1167. https://doi.org/10.1080/22221751.2019.1648183
Chagvardieff A, Persico N, Marmillot C et al (2018) Prospective comparative study of characteristics associated with influenza A and B in adults. Med Mal Infect 48:180–187. https://doi.org/10.1016/j.medmal.2017.11.007
Su S, Chaves SS, Perez A et al (2014) Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis 59:252–255. https://doi.org/10.1093/cid/ciu269
Jain S, Self WH, Wunderink RG et al (2015) Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. https://doi.org/10.1093/cid/ciu269
Chen L, Zhou F, Li H et al (2018) Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: a retrospective multicentre survey. BMJ Open 8:e018709. https://doi.org/10.1136/bmjopen-2017-018709
Lytras T, Mouratidou E, Andreopoulou A et al (2019) Effect of early Oseltamivir treatment on mortality in critically ill patients with different types of influenza: a multiseason cohort study. Clin Infect Dis 69:1896–1902. https://doi.org/10.1093/cid/ciz101
Muthuri SG, Venkatesan S, Myles PR et al (2016) Impact of neuraminidase inhibitors on influenza a(H1N1)pdm09-related pneumonia: an individual participant data meta-analysis. Influenza Other Respir Viruses 10:192–204. https://doi.org/10.1111/irv.12363
Thiese MS (2014) Observational and interventional study design types; an overview. Biochem Med (Zagreb) 24:199–210. https://doi.org/10.11613/BM.2014.022
Ikematsu H, Kawai N, Iwaki N et al (2016) In vitro neuraminidase inhibitory concentration (IC50) of four neuraminidase inhibitors against clinical isolates of the influenza viruses circulating in the 2010-2011 to 2014-2015 Japanese influenza seasons. J Infect Chemother 22:599–604. https://doi.org/10.1016/j.jiac.2016.06.002
Ishiguro N, Koseki N, Kaiho M et al (2018) Clinical effectiveness of four neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir, and peramivir) for children with influenza a and B in the 2014-2015 to 2016-2017 influenza seasons in Japan. J Infect Chemother 24:449–457. https://doi.org/10.1016/j.jiac.2018.01.013
Kawai N, Ikematsu H, Iwaki N et al (2006) A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003-2004 and 2004-2005 influenza seasons. Clin Infect Dis 43:439–444. https://doi.org/10.1086/505868
Ikematsu H, Kawai N, Iwaki N et al (2018) Duration of fever and other symptoms after the inhalation of laninamivir octanoate hydrate in the 2016/17 Japanese influenza season; comparison with the 2011/12 to 2015/16 seasons. J Infect Chemother 24:718–724. https://doi.org/10.1016/j.jiac.2018.04.013
Marty FM, Vidal-Puigserver J, Clark C et al (2017) Intravenous zanamivir or oral oseltamivir for hospitalised patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir Med 5:135–146. https://doi.org/10.1016/S2213-2600(16)30435-0
Lee N, Choi KW, Chan PK et al (2010) Outcomes of adults hospitalised with severe influenza. Thorax 65:510–515. https://doi.org/10.1136/thx.2009.130799
Fairchok MP, Chen WJ, Arnold JC et al (2015) Neuraminidase inhibitor therapy in a military population. J Clin Virol 67:17–22. https://doi.org/10.1016/j.jcv.2015.03.018
Hagau N, Slavcovici A, Gonganau DN et al (2010) Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 14:R203. https://doi.org/10.1186/cc9324
Zheng BJ, Chan KW, Lin YP et al (2008) Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza a/H5N1 virus. Proc Natl Acad Sci U S A 105:8091–8096. https://doi.org/10.1073/pnas.0711942105
Louie JK, Yang S, Acosta M et al (2012) Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis 55:1198–1204. https://doi.org/10.1093/cid/cis636
Kossyvakis A, Mentis AA, Tryfinopoulou K et al (2017) Antiviral susceptibility profile of influenza A viruses; keep an eye on immunocompromised patients under prolonged treatment. Eur J Clin Microbiol Infect Dis 36:361–371. https://doi.org/10.1007/s10096-016-2809-3
Pascua PNQ, Mostafa HH, Marathe BM et al (2017) Pathogenicity and peramivir efficacy in immunocompromised murine models of influenza B virus infection. Sci Rep 7:7345. https://doi.org/10.1038/s41598-017-07433-z
Moreno G, Rodríguez A, Reyes LF et al (2018) Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 44:1470–1482. https://doi.org/10.1007/s00134-018-5332-4
Dobson J, Whitley RJ, Pocock S et al (2015) Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 385:1729–1737. https://doi.org/10.1016/S0140-6736(14)62449-1
Jefferson T, Jones MA, Doshi P et al (2014) Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 4:CD008965. https://doi.org/10.1002/14651858.CD008965.pub4
Ebell MH, Call M, Shinholser J (2013) Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract 30:125–133. https://doi.org/10.1093/fampra/cms059
National Health Commission of the People’s Republic of China. Clinical guidance for patients with influenza infection. http://wwwnhcgovcn/xxgk/pages/viewdocumentjsp?%20dispatchDate=&staticUrl=%2Fyzygj%2Fpqt%2F201811%2Fdd748b43df0640e0bf%2033c526ca8c9ddfshtml&wenhao=%E5%9B%BD%E5%8D%AB%E5%8A%9E%20%E5%8C%BB%E5%87%BD%E3%80%942018%E3%80%951020%E5%8F%B7%20&utitle=%E5%85%B3%E4%BA%8E%E8%BF%9B%E4%B8%80%E6%AD%%20A5%E5%8A%A0%E5%BC%BA%E6%B5%81%E8%A1%8C%E6%80%A7%E6%%2084%9F%E5%86%92%E5%8C%BB%E7%96%97%E5%B7%A5%E4%BD%9C%E7%20%9A%84%E9%80%9A%E7%9F%A5&topictype=&topic=&publishedOrg=%E5%%208C%BB%E6%94%BF%E5%8C%BB%E7%AE%A1%E5%B1%80&indexNum=%20000013610%2F2018–00308&manuscriptId=dd748b43df0640e0bf33c526ca%208c9ddf
Uyeki TM, Bernstein HH, Bradley JS et al (2019) Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak Management of Seasonal Influenza A. Clin Infect Dis 68:e1–e47. https://doi.org/10.1093/cid/ciy874
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
The study design was approved by the Ethics Committee of Chao-Yang Hospital (No.2015–86).
Informed consent
Not applicable to this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Han, X., Li, Y. et al. Impact of early neuraminidase inhibitor treatment on clinical outcomes in patients with influenza B-related pneumonia: a multicenter cohort study. Eur J Clin Microbiol Infect Dis 39, 1231–1238 (2020). https://doi.org/10.1007/s10096-020-03835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-03835-6