Abstract
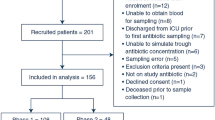
Therapeutic drug monitoring (TDM) of teicoplanin is aimed at minimizing the clinical impact of pharmacokinetic variability; however, its benefits are still being defined. We performed a retrospective study of teicoplanin TDM focusing on the dose-serum concentration relationship and clinical outcomes in a clinical setting. From January 2017 to December 2018, patients receiving teicoplanin ≥ 72 h with TDM were enrolled. Patients were divided into three groups: non-loading (NL) group, low-dose loading (LD) group (loading dose < 9 mg/kg), and high-dose loading (HD) group (≥ 9 mg/kg). Serum teicoplanin trough concentration (Cmin) and adverse events (AEs) were evaluated in each regimen. A subgroup of patients with bacteremia was analyzed to evaluate clinical efficacy. Among 65 patients, 12, 18, and 35 were grouped in NL, LD, and HD, respectively. Achievement rates of Cmin > 20 mg/L within 10 days were significantly different among the groups (25.0%, 38.9%, and 68.6% in the NL, LD, and HD groups, respectively; P = 0.014). Fourteen patients (21.5%) had AEs, and higher Cmin over 10 days (adjusted odds ratio 2.08 per every 20 mg/L increases, 95% CI 1.13–3.84, P = 0.019) and age ≥ 65 years (P = 0.009) were identified as independent risk factors. In the subgroup analysis, HD regimen (P = 0.050) and high mean Cmin over 10 days (P = 0.025) were significantly associated with treatment success. Although HL regimen could achieve Cmin targets and improve clinical outcome during teicoplanin treatment, high Cmin was associated with AEs during treatment. Routine TDM can be helpful to optimize teicoplanin administration.


Similar content being viewed by others
References
Tobin CM, Lovering AM, Sweeney E, MacGowan AP (2010) Analyses of teicoplanin concentrations from 1994 to 2006 from a UK assay service. J Antimicrob Chemother 65(10):2155–2157. https://doi.org/10.1093/jac/dkq266
Outman WR, Nightingale CH, Sweeney KR, Quintiliani R (1990) Teicoplanin pharmacokinetics in healthy volunteers after administration of intravenous loading and maintenance doses. Antimicrob Agents Chemother 34(11):2114–2117. https://doi.org/10.1128/aac.34.11.2114
Smithers JA, Kulmala HK, Thompson GA, Antony KK, Lewis EW, Ruberg SJ, Kenny MT, Dulworth JK, Brackman MA (1992) Pharmacokinetics of teicoplanin upon multiple-dose intravenous administration of 3, 12, and 30 milligrams per kilogram of body weight to healthy male volunteers. Antimicrob Agents Chemother 36(1):115–120. https://doi.org/10.1128/aac.36.1.115
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J (2011) The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50(2):99–110. https://doi.org/10.2165/11539220-000000000-00000
Nah SY, Im JH, Yeo JY, Baek JH, Kim CW, Nam MS, Lee HK, Chung MH, Lee JS (2014) Therapeutic drug concentrations of teicoplanin in clinical settings. Infection & chemotherapy 46(1):35–41. https://doi.org/10.3947/ic.2014.46.1.35
Byrne CJ, Roberts JA, McWhinney B, Fennell JP, O’Byrne P, Deasy E, Egan S, Desmond R, Enright H, Ryder SA, D’Arcy DM, McHugh J (2017) Variability in trough total and unbound teicoplanin concentrations and achievement of therapeutic drug monitoring targets in adult patients with hematological malignancy. Antimicrob Agents Chemother 61(6):e02466. https://doi.org/10.1128/AAC.02466-16
Ahn BJ, Yim DS, Lee DG, Kwon JC, Kim SH, Choi SM (2011) Teicoplanin dosing strategy for treatment of Staphylococcus aureus in Korean patients with neutropenic fever. Yonsei Med J 52(4):616–623. https://doi.org/10.3349/ymj.2011.52.4.616
Harding I, MacGowan AP, White LO, Darley ES, Reed V (2000) Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J Antimicrob Chemother 45(6):835–841. https://doi.org/10.1093/jac/45.6.835
Byrne CJ, Egan S, Fennell JP, O’Byrne P, Enright H, Deasy E, Ryder SA, D’Arcy DM, McHugh J (2015) Teicoplanin use in adult patients with haematological malignancy: exploring relationships between dose, trough concentrations, efficacy and nephrotoxicity. Int J Antimicrob Agents 46(4):406–412. https://doi.org/10.1016/j.ijantimicag.2015.05.019
Nakamura A, Takasu O, Sakai Y, Sakamoto T, Yamashita N, Mori S, Morita T, Nabeta M, Hirayu N, Yoshiyama N, Moroki M, Tashiro K, Kannae M (2015) Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J Infect Chemother 21(6):449–455. https://doi.org/10.1016/j.jiac.2015.02.002
Ueda T, Takesue Y, Nakajima K, Ichki K, Wada Y, Komatsu M, Tsuchida T, Takahashi Y, Ishihara M, Kimura T, Uchino M, Ikeuchi H (2014) High-dose regimen to achieve novel target trough concentration in teicoplanin. J Infect Chemother 20(1):43–47. https://doi.org/10.1016/j.jiac.2013.08.006
Wilson AP (2000) Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 39(3):167–183. https://doi.org/10.2165/00003088-200039030-00001
Greenberg RN (1990) Treatment of bone, joint, and vascular-access-associated gram-positive bacterial infections with teicoplanin. Antimicrob Agents Chemother 34(12):2392–2397. https://doi.org/10.1128/aac.34.12.2392
Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354(23):2473–2483. https://doi.org/10.1056/NEJMra054415
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama 286(14):1754–1758. https://doi.org/10.1001/jama.286.14.1754
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
U.S. Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [accessed 1 November 2018]
Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356(9237):1255–1259. https://doi.org/10.1016/S0140-6736(00)02799-9
Seifert H (2009) The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 48(Suppl 4):S238–S245. https://doi.org/10.1086/598188
Jung J, Lee K, Oh J, Choi R, Woo HI, Park HD, Kang CI, Kim YJ, Lee SY (2019) Therapeutic drug monitoring of teicoplanin using an LC-MS/MS method: analysis of 421 measurements in a naturalistic clinical setting. J Pharm Biomed Anal 167:161–165. https://doi.org/10.1016/j.jpba.2019.02.001
Verbist L, Tjandramaga B, Hendrickx B, Van Hecken A, Van Melle P, Verbesselt R, Verhaegen J, De Schepper PJ (1984) In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother 26(6):881–886. https://doi.org/10.1128/aac.26.6.881
Sato M, Chida K, Suda T, Muramatsu H, Suzuki Y, Hashimoto H, Gemma H, Nakamura H (2006) Recommended initial loading dose of teicoplanin, established by therapeutic drug monitoring, and outcome in terms of optimal trough level. J Infect Chemother 12(4):185–189. https://doi.org/10.1007/s10156-006-0446-y
Zhou L, Gao Y, Cao W, Liu J, Guan H, Zhang H, Shi Y, Lv W, Cheng L (2018) Retrospective analysis of relationships among the dose regimen, trough concentration, efficacy, and safety of teicoplanin in Chinese patients with moderate-severe Gram-positive infections. Infect Drug Resist 11:29–36. https://doi.org/10.2147/IDR.S146961
Matthews PC, Chue AL, Wyllie D, Barnett A, Isinkaye T, Jefferies L, Lovering A, Scarborough M (2014) Increased teicoplanin doses are associated with improved serum levels but not drug toxicity. The Journal of infection 68(1):43–49. https://doi.org/10.1016/j.jinf.2013.08.018
Lee CH, Tsai CY, Li CC, Chien CC, Liu JW (2015) Teicoplanin therapy for MRSA bacteraemia: a retrospective study emphasizing the importance of maintenance dosing in improving clinical outcomes. J Antimicrob Chemother 70(1):257–263. https://doi.org/10.1093/jac/dku335
Davey PG, Williams AH (1991) A review of the safety profile of teicoplanin. J Antimicrob Chemother 27 Suppl_B:69–73. https://doi.org/10.1093/jac/27.suppl_b.69
Byrne CJ, Egan S, D’Arcy DM, O’Byrne P, Deasy E, Fennell JP, Enright H, McHugh J, Ryder SA (2014) Teicoplanin usage in adult patients with haematological malignancy in the UK and Ireland: is there scope for improvement? Eur J Hosp Pharm 21(5):301–305. https://doi.org/10.1136/ejhpharm-2013-000412
Acknowledgments
The authors thank Junsang Yoo who helped with the graphs and Hyo Jung Park, MS, who provided advice for TDM analysis.
Funding
This material is based upon work supported by the Ministry of Trade, Industry, & Energy (MOTIE, Korea) under the Industrial Technology Innovation Program (No. 10080648: Antibiotics monitoring point-for-care test).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study was approved by the local ethical research committee (IRB number 2018-07-162-003).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, SH., Kang, CI., Huh, K. et al. Evaluating the optimal dose of teicoplanin with therapeutic drug monitoring: not too high for adverse event, not too low for treatment efficacy. Eur J Clin Microbiol Infect Dis 38, 2113–2120 (2019). https://doi.org/10.1007/s10096-019-03652-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03652-6