Abstract
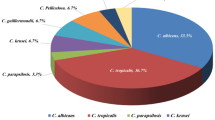
To evaluate the association between fluconazole exposure parameters and clinical outcomes in patients with candidemia. We retrospectively included all adults with candidemia in a single center from January 2009 to December 2017, treated initially with fluconazole for fluconazole-susceptible candidemia. We assessed the association between fluconazole exposure parameters and 30-day mortality or 14-day clinical failure, a composite of mortality at day 14 or persistent candidemia ≥ 72 h, in all patients and in patients with C. glabrata candidemia. During the study period, 158 patients fulfilled the inclusion criteria. Main species were C. albicans 66 (41.8%), C. glabrata 35 (22.2%), and C. parapsilosis 31 (19.6%). Sixty patients (38%) died within 30 days. Sixty-one patients (38.6%) experienced 14-day failure. In 30-day survivors, the median AUC24/MIC was 2279 [398, 5989] versus 1764 [238, 6714] h in non-survivors, p = 0.75. Median fluconazole MIC was 0.75 [0.25, 4] and 1 [0.22, 5.50] mg/L, p = 0.54, respectively. Similar non-significant differences were found for other fluconazole exposure parameters and in the 14-day clinical failure analysis. For C. glabrata, a higher AUC24/MIC was observed among 30-day survivors with a median of 230 [77, 539] compared to 96 [75, 164] h in non-survivors, p = 0.008, in parallel with a trend for lower MIC values (median 7 [1, 2] versus 16 [8, 24] mg/L, p = 0.06, respectively). Currently used fluconazole dosing has no association with clinical outcome in Candida with low MIC values. For Candida species with high MICs, attention to dosing is needed.
Similar content being viewed by others

References
Clinical and laboratory standards institute reference method for broth dilution antifungal susceptibility testing of yeasts: Fourth informational supplement M27-S4. Wayne, PA: USACLSI; 2012
Koga-Ito CY, Lyon JP, de Resende MA Comparison between E-test and CLSI broth microdilution method for antifungal susceptibility testing of Candida albicans oral isolates. Rev Inst Med Trop Sao Paulo [cited 2019 7];50:7–10. http://www.ncbi.nlm.nih.gov/pubmed/18327480
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al (2015) Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis [cited 2019 2];62:civ933. http://www.ncbi.nlm.nih.gov/pubmed/26679628 https://doi.org/10.1093/cid/civ933
Fluconazole rationale for the EUCAST clinical breakpoints, version 2.0. 2013 [cited 2019 24]. http://www.eucast.org
European committee on antimicrobial susceptibility testing antifungal agents. Breakpoint tables for interpretation of MICs. Version 7.0. 2014;0–4
Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH (2005) Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother [cited 2018 19];49:3171–7. http://www.ncbi.nlm.nih.gov/pubmed/16048920 https://doi.org/10.1128/AAC.49.8.3171-3177.2005
Pai MP, Turpin RS, Garey KW (2007) Association of fluconazole area under the concentration-time curve/mic and dose/mic ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 1 [cited 2018 18];51:35–9. http://www.ncbi.nlm.nih.gov/pubmed/17101684 https://doi.org/10.1128/AAC.00474-06
Rodríguez-Tudela JL, Almirante B, Rodríguez-Pardo D, Laguna F, Donnelly JP, Mouton JW, et al (2007) Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob Agents Chemother [cited 2018 19];51:3599–604. http://www.ncbi.nlm.nih.gov/pubmed/17646421 https://doi.org/10.1128/AAC.00296-07
Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR (2008) Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob Agents Chemother [cited 2018 19];52:3022–3028. http://www.ncbi.nlm.nih.gov/pubmed/18591269 https://doi.org/10.1128/AAC.00116-08
Brosh-Nissimov T, Ben-Ami R (2015) Differential association of fluconazole dose and dose/MIC ratio with mortality in patients with Candida albicans and non-albicans bloodstream infection. Clin Microbiol Infect [cited 2018 19]; 21:1011–1017. https://doi.org/10.1016/j.cmi.2015.07.005 https://doi.org/10.1016/j.cmi.2015.07.005
Fernández-Ruiz M, Guinea J, Lora-Pablos D, Zaragoza Ó, Puig-Asensio M, Almirante B, et al (2017) Impact of fluconazole susceptibility on the outcome of patients with candidaemia: data from a population-based surveillance. Clin Microbiol Infect [cited 2018 19];23:672.e1–672.e11. https://www.sciencedirect.com/science/article/pii/S1198743X17300472?via%3Dihub https://doi.org/10.1016/J.CMI.2017.01.014
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al (2017) Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem [cited 2019 30];53:766–72. http://www.ncbi.nlm.nih.gov/pubmed/17332152 https://doi.org/10.1373/clinchem.2006.077180
Sobue S, Tan K, Layton G, Leclerc V, Weil A (2004) The effects of renal impairment on the pharmacokinetics and safety of fosfluconazole and fluconazole following a single intravenous bolus injection of fosfluconazole. Br J Clin Pharmacol [cited 2019 30];57:773–784. http://doi.wiley.com/10.1111/j.1365-2125.2004.02073.x https://doi.org/10.1111/j.1365-2125.2004.02073.x
Siqueira RA, Doi AM, de Petrus Crossara PP, Koga PCM, Marques AG, Nunes FG, et al (2018) Evaluation of two commercial methods for the susceptibility testing of Candida species: Vitek 2® and Sensititre YeastOne®. Rev Iberoam Micol [cited 2019 7];35:83–7. http://www.ncbi.nlm.nih.gov/pubmed/29580699 https://doi.org/10.1016/j.riam.2017.11.001
Pfaller MA, Diekema DJ, Sheehan DJ (2006) Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev [cited 2019 9];19:435–47. http://www.ncbi.nlm.nih.gov/pubmed/16614256 https://doi.org/10.1128/CMR.19.2.435-447.2006
Kaur R, Dhakad M, Goyal R, Haque A, Mukhopadhyay G (2016) Identification and antifungal susceptibility testing of Candida species: a comparison of Vitek-2 system with conventional and molecular methods. J Glob Infect Dis [cited 2019 7];8:139. http://www.ncbi.nlm.nih.gov/pubmed/27942193 https://doi.org/10.4103/0974-777X.192969
Acknowledgments
The study was presented in the 29th European Congress of Clinical Microbiology and Infectious Diseases that was held in Amsterdam, Netherlands, April 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study was approved by the ethics committee at Rambam Hospital.
Informed consent
Not required; not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Ghanem-Zoubi, N., Qasum, M., Khoury, J. et al. The association between fluconazole dose and MIC with mortality and persistence in candidemia. Eur J Clin Microbiol Infect Dis 38, 1773–1780 (2019). https://doi.org/10.1007/s10096-019-03611-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03611-1