Abstract
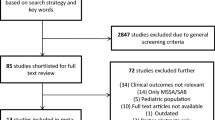
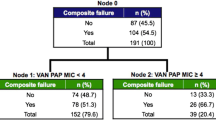
Vancomycin (VAN) minimum inhibitory concentrations (MICs) at the upper end of the susceptible range for Staphylococcus aureus (S. aureus), as measured by the Etest method, have been associated with poor clinical outcomes of S. aureus bloodstream infections, as has the isolate’s genetic background. Here, we assessed the impact of VAN MICs, as determined by a broth microdilution method (BMD) that incorporates incremental VAN concentrations between the conventional log2 dilutions, isolate susceptibility to killing by human phagocytes, acting as a surrogate marker for bacterial cell wall thickness, and S. aureus genetic composition, on the development of complicated S. aureus bacteremia (SAB). We carried out a retrospective, observational single-center cohort study of 148 consecutive patients with SAB caused by methicillin-susceptible (MSSA) isolates (n = 113) or methicillin-resistant (MRSA) isolates (n = 35). S. aureus isolates were genotyped using a commercially available DNA microarray. Overall, VAN MICs of S. aureus isolates taken from complicated and uncomplicated SAB were comparable, irrespective of the testing method (P = 0.19 with BMD, and P = 0.94 with Etest). Likewise, S. aureus isolates in both comparison groups had the same susceptibility to killing by human phagocytes (P = 0.5). Among the genes screened by the S. aureus DNA array, only Sec and Sel were differentially present among S. aureus isolates in both groups (overrepresented in those causing complications) and their presence was associated independently with complicated SAB in multivariate models adjusted for potentially relevant clinical covariates. Separate analysis of MSSA SAB episodes yielded similar results.




Similar content being viewed by others
References
Soriano A, Marco F, Martínez JA, Pisos E, Almela M, Dimova VP et al (2008) Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46:193–200
Pea F, Viale P (2009) Is the minimum inhibitory concentration of vancomycin an infallible predictor of the clinical outcome of Staphylococcus aureus bacteremia treated with vancomycin? Clin Infect Dis 49:642–643
Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV et al (2011) Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 204:340–347
Aguado JM, San-Juan R, Lalueza A, Sanz F, Rodríguez-Otero J, Gómez-Gonzalez C et al (2011) High vancomycin MIC and complicated methicillin-susceptible Staphylococcus aureus bacteremia. Emerg Infect Dis 17:1099–1102
Holland TL, Fowler VG Jr (2011) Vancomycin minimum inhibitory concentration and outcome in patients with Staphylococcus aureus bacteremia: pearl or pellet? J Infect Dis 204:329–331
Aguado JM, San-Juan R, Fernández-Ruiz M, Chaves F (2012) Role of high vancomycin minimum inhibitory concentration in the outcome of methicillin-susceptible Staphylococcus aureus bacteremia. J Infect Dis 205:1024–1025
Gould IM (2012) Vancomycin minimum inhibitory concentrations and outcome in patients with severe Staphylococcus aureus infection. J Infect Dis 205:864–865
Cervera C, Castañeda X, García de la María C, del Rio A, Moreno A, Soy D et al (2014) Effect of vancomycin minimal inhibitory concentration on the outcome of methicillin-susceptible Staphylococcus aureus endocarditis. Clin Infect Dis 58:1668–1675
Jacob JT, Diaz Granados CA (2013) High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: a meta-analysis. Int J Infect Dis 17:e93–e100
Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME (2014) Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564
Rojas L, Bunsow E, Muñoz P, Cercenado E, Rodríguez-Créixems M, Bouza E (2012) Vancomycin MICs do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. J Antimicrob Chemother 67:1760–1768
López-Cortés LE, Velasco C, Retamar P, del Toro MD, Gálvez-Acebal J, de Cueto M et al (2015) Is reduced vancomycin susceptibility a factor associated with poor prognosis in MSSA bacteraemia? J Antimicrob Chemother 70:2652–2660
Chen SY, Liao CH, Wang JL, Chiang WC, Lai MS, Chie WC et al (2014) Method-specific performance of vancomycin MIC susceptibility tests in predicting mortality of patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 69:211–218
Shoji H, Maeda M, Shirakura T, Takuma T, Ugajin K, Fukuchi K et al (2015) More accurate measurement of vancomycin minimum inhibitory concentration indicates poor outcomes in meticillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 46:532–537
Adani S, Bhowmick T, Weinstein MP, Narayanan N (2018) Impact of vancomycin MIC on clinical outcomes of patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin at an institution with suppressed MIC reporting. Antimicrob Agents Chemother 62:e02512–e02517
Bouiller K, Laborde C, Aho SL, Hocquet D, Pechinot A, Le Moing V et al (2018) No effect of vancomycin MIC ≥ 1.5 mg/L on treatment outcome in methicillin-susceptible Staphylococcus aureus bacteraemia. Int J Antimicrob Agents 51:721–726
Fernández-Hidalgo N, Ribera A, Larrosa MN, Viedma E, Origüen J, de Alarcón A et al (2018) Impact of Staphylococcus aureus phenotype and genotype on the clinical characteristics and outcome of infective endocarditis. A multicentre, longitudinal, prospective, observational study. Clin Microbiol Infect 24:985–991
San-Juan R, Fernández-Ruiz M, Gasch O, Camoez M, López-Medrano F, Domínguez MÁ et al (2017) High vancomycin MICs predict the development of infective endocarditis in patients with catheter-related bacteraemia due to methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 72:2102–2109
Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B et al (2013) Predictive factors for mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infection: impact on outcome of host, microorganism and therapy. Clin Microbiol Infect 19:1049–1057
Sullivan SB, Austin ED, Stump S, Mathema B, Whittier S, Lowy FD et al (2017) Reduced vancomycin susceptibility of methicillin-susceptible Staphylococcus aureus has no significant impact on mortality but results in an increase in complicated infection. Antimicrob Agents Chemother 61:e00316–e00317
Falcón R, Madrid S, Tormo N, Casañ C, Albert E, Gimeno C et al (2015) Intra- and interinstitutional evaluation of an Etest for vancomycin minimum inhibitory concentration measurement in Staphylococcus aureus blood isolates. Clin Infect Dis 61:1490–1492
Falcón R, Mateo EM, Talaya A, Giménez E, Vinuesa V, Clari MÁ et al (2017) Reproducible measurement of vancomycin MICs within the susceptible range in Staphylococcus aureus by a broth microdilution method with a “quasi-continuum” gradient of antibiotic concentrations. Eur J Clin Microbiol Infect Dis 36:2355–2360
Cui L, Murakami H, Kuwahara-Arai K, Hanaki H, Hiramatsu K (2000) Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob Agents Chemother 44:2276–2285
Cui L, Ma X, Sato K, Tenover FC, Mamizuka EM, Gemmell CG et al (2003) Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41:5–14
Falcón R, Martínez A, Albert E, Madrid S, Oltra R, Giménez E et al (2016) High vancomycin MICs within the susceptible range in Staphylococcus aureus bacteraemia isolates are associated with increased cell wall thickness and reduced intracellular killing by human phagocytes. Int J Antimicrob Agents 47:343–350
Lalani T, Federspiel JJ, Boucher HW, Rude TH, Bae IG, Rybak MJ et al (2008) Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol 46:2890–2896
Viedma E, Sanz F, Orellana MA, San Juan R, Aguado JM, Otero JR et al (2014) Relationship between agr dysfunction and reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus causing bacteraemia. J Antimicrob Chemother 69:51–58
Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV et al (2014) Genetic and molecular predictors of high vancomycin MIC in Staphylococcus aureus bacteremia isolates. J Clin Microbiol 52:3384–3393
San-Juan R, Pérez-Montarelo D, Viedma E, Lalueza A, Fortún J, Loza E et al (2017) Pathogen-related factors affecting outcome of catheter-related bacteremia due to methicillin-susceptible Staphylococcus aureus in a Spanish multicenter study. Eur J Clin Microbiol Infect Dis 36:1757–1765
Kruzel MC, Lewis CT, Welsh KJ, Lewis EM, Dundas NE, Mohr JF et al (2011) Determination of vancomycin and daptomycin MICs by different testing methods for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 49:2272–2273
Muñoz-Cobo B, Sancho-Tello S, Costa E, Bravo D, Torregrosa I, de Lomas JG et al (2011) Differences in vancomycin minimum inhibitory concentrations for Staphylococcus aureus obtained with the automated Phoenix™ system, the clinical and laboratory standards institute broth microdilution method and the standard Etest. Int J Antimicrob Agents 37:278–279
Charlton CL, Hindler JA, Turnidge J, Humphries RM (2014) Precision of vancomycin and daptomycin MICs for methicillin-resistant Staphylococcus aureus and effect of subculture and storage. J Clin Microbiol 52:3898–3905
Baxi SM, Clemenzi-Allen A, Gahbauer A, Deck D, Imp B, Vittinghoff E et al (2016) Vancomycin MIC does not predict 90-day mortality, readmission, or recurrence in a prospective cohort of adults with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 60:5276–5284
Fowler VG Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL et al (2007) Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196:738–747
Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS (2009) Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 199:201–208
Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH et al (2011) Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis 204:704–713
Nethercott C, Mabbett AN, Totsika M, Peters P, Ortiz JC, Nimmo GR et al (2013) Molecular characterization of endocarditis-associated Staphylococcus aureus. J Clin Microbiol 51:2131–2138
Bouchiat C, Moreau K, Devillard S, Rasigade JP, Mosnier A, Geissmann T et al (2015) Staphylococcus aureus infective endocarditis versus bacteremia strains: subtle genetic differences at stake. Infect Genet Evol 36:524–530
Wong H, Watt C, Elsayed S, John M, Johnson G, Katz K et al (2014) Characterization of methicillin-resistant Staphylococcus aureus isolates from patients with persistent or recurrent bacteremia. Can J Infect Dis Med Microbiol 25:83–86
Lilje B, Rasmussen RV, Dahl A, Stegger M, Skov RL, Fowler VG, et al. (2017) Whole-genome sequencing of bloodstream Staphylococcus aureus isolates does not distinguish bacteraemia from endocarditis. Microb Genom. https://doi.org/10.1099/mgen.0.000138
Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM (2013) Staphylococcal and streptococcal superantigen exotoxins. Clin Microbiol Rev 26:422–447
Li H, Llera A, Malchiodi EL, Mariuzza RA (1999) The structural basis of T cell activation by superantigens. Annu Rev Immunol 17:435–466
Salgado-Pabón W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR et al. (2013) Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. MBio. https://doi.org/10.1128/mBio.00494-13
Acknowledgements
Estela Giménez holds a Río Hortega research contract from the Carlos III Health Institute (Ref. CM16/00200). Eva María Mateo is grateful to the Ministry of Economy and Competitiveness (MINECO, Spanish Government) for the “Juan de la Cierva” postdoctoral contract (Ref. FJCI-2015-25992).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Transparency declarations
None to declare
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Falcón, R., Mateo, E., Oltra, R. et al. Vancomycin MICs and risk of complicated bacteremia by glycopeptide-susceptible Staphylococcus aureus. Eur J Clin Microbiol Infect Dis 38, 903–912 (2019). https://doi.org/10.1007/s10096-019-03500-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03500-7