Abstract
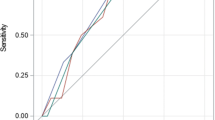
The management of bacteremia by carbapenem-resistant Gram-negative bacteria (CRGNB) necessitates a surrogate marker for response to treatment. We developed a prognostic score of bacteremia resolution using a test and a validation cohort. In the test cohort, five protein biomarkers were measured in serial daily serum samples from 39 patients with ventilator-associated pneumonia (VAP) and CRGNB bacteremia. Receiver operator characteristic curves were designed to identify cut-off of over-time changes that were associated with more than 80% specificity for resolution of bacteremia. The developed score was validated in a cohort of 24 patients mostly with primary bacteremia by carbapenem-resistant enterobacteria (CRE). Among the five tested biomarkers, only procalcitonin (PCT) was associated with resolution of bacteremia. More precisely, resolved bacteremia was considered if at least one of three situations occurred: (a) PCT on day 2 was decreased more than 30% and PCT on day 4 was below 0.5 ng/ml; (b) PCT on day 4 was decreased more than 40% and PCT on day 4 was below 0.5 ng/ml; and (c) PCT on day 2 was decreased more than 30% and PCT on day 4 was decreased more than 40%. Sensitivity, specificity, and positive and negative predictive values of the score were 66.7%, 83.3%, 90.0%, and 52.6% respectively. This score was fully validated (p values of comparison between the cohorts 0.623). The developed score is highly predictive of resolution of bacteremia by CRGNB. A prospective clinical study is mandatory to validate the results.


Similar content being viewed by others
References
Ramos-Castañeda JA, Ruano-Ravina A, Barbosa-Lorenzo R, Paillier-Gonzalez JE, Saldaña-Campos JC, Salinas DF, Lemos-Luengas EV (2018) Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: systematic review and meta-analysis. J Infect 76:438–448
van Loon K, Voor In’t Holt AF, Vos MC (2017) A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant enterobacteriaceae. Antimicrob Agents Chemother 62:e01730–e01717
Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G (2018) Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 24:133–144
Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, Zervakis D, Baziaka F, Koronaios A, Antonopoulou A, Markaki V, Koutoukas P, Papadomichelakis E, Tsaganos T, Armaganidis A, Koussoulas V, Kotanidou A, Roussos C, Giamarellou H (2008) Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin Infect Dis 46:1157–1164
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Régnier B, Brun-Buisson C, Chastre J, Wolff M (2010) Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375:463–474
de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC, Kemperman H, van den Elsen MJ, Schouten JA, Streefkerk JO, Krabbe HG, Kieft H, Kluge GH, van Dam VC, van Pelt J, Bormans L, Otten MB, Reidinga AC, Endeman H, Twisk JW, van de Garde EMW, de Smet AMGA, Kesecioglu J, Girbes AR, Nijsten MW, de Lange DW (2016) Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 16:819–827
Georgopoulou AP, Savva A, Giamarellos-Bourboulis EJ, Georgitsi M, Raftogiannis M, Antonakos N, Apostolidou E, Carrer DP, Dimopoulos G, Economou A, Efthymiou G, Galanakis N, Galani L, Gargalianos P, Karaiskos I, Katsenos C, Kavatha D, Koratzanis E, Labropoulos P, Lada M, Nakos G, Paggalou E, Panoutsopoulos G, Paraschos M, Pavleas I, Pontikis K, Poulakou G, Prekates A, Sybardi S, Theodorakopoulou M, Trakatelli C, Tsiaoussis P, Gogos C, Giamarellou H, Armaganidis A, Meisner M (2011) Early changes of procalcitonin may advise about prognosis and appropriateness of antimicrobial therapy in sepsis. J Crit Care 26:331.e1–331.e7
Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E (2013) Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care 17:R115
Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, Marsch S, Azzola A, Rakic J, Mueller B, Tamm M (2009) Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 34:1364–1375
Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB (2016) Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762
Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J (2016) Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389
Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, Vazquez J, Zaitsev V, Bidair M, Chorvat E, Dragoescu PO, Fedosiuk E, Horcajada JP, Murta C, Sarychev Y, Stoev V, Morgan E, Fusaro K, Griffith D, Lomovskaya O, Alexander EL, Loutit J, Dudley MN, Giamarellos-Bourboulis EJ (2018) Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: The TANGO I randomized clinical trial. JAMA 319:788–799
Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ (2017) Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883–e00817
van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG Jr, Paterson DL, Bonomo RA, Evans S (2018) Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Infect Dis 66:163–171
Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A (2014) Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328
Quartin AA, Scerpella EG, Puttagunta S, Kett DH (2013) A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 13:561
Funding
Part of the study was supported by an unrestricted educational grant provided by Merck to the University of Athens [grant 70/3/11471]. The funders did not have any role in study design, analysis, and interpretation of data and drafting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EJ Giamarellos-Bourboulis has received honoraria (paid to the University of Athens) from AbbVie USA, Abbott CH, Biotest Germany, Brahms GmbH, InflaRx GmbH, the Medicines Company, MSD Greece, and XBiotech Inc. He has received independent educational grants from AbbVie, Abbott, Astellas Pharma, AxisShield, bioMérieux Inc., InflaRx GmbH, the Medicines Company, and XBiotech Inc. He has received funding from the FrameWork 7 program HemoSpec and from the Horizon2020 Marie-Curie project European Sepsis Academy (granted to the National and Kapodistrian University of Athens).
M Bassetti serves on scientific advisory boards for Angelini, AstraZeneca, Bayer, Cubist, Pfizer, Menarini, MSD, Nabriva, Paratek, Roche, Shionogi, Tetraphase, The Medicine Company, and Astellas Pharma Inc.; has received funding for travel or speaker honoraria from Algorithm, Angelini, Astellas Pharma Inc., AstraZeneca, Cubist, Pfizer MSD, Gilead Sciences, Menarini, Novartis, Ranbaxy, and Teva. The other authors declare no conflict of interest.
Ethical approval
The study was approved by the Ethics Committees of the hospitals of the participating study sites and by the National Organization of Medicines of Greece (http://clinicaltrials.gov: registration number NCT00297674).
Informed consent
Patients were enrolled after written consent provided by first-degree relatives.
Rights and permissions
About this article
Cite this article
Giamarellos-Bourboulis, E.J., Kotsaki, A., Routsi, C. et al. A prognostic score for the resolution of bacteremia by Gram-negative bacteria resistant to carbapenems. Eur J Clin Microbiol Infect Dis 37, 2083–2089 (2018). https://doi.org/10.1007/s10096-018-3342-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3342-3