Abstract
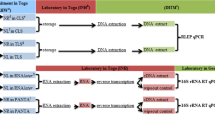
To verify if the hard palate mucosa can be a site of relevance in the early molecular detection of Mycobacterium leprae in leprosy cases and their household contacts and if there is a correlation of results in nasal swab with those of the scraping of the palate mucosa. The quantitative polymerase chain reaction technique was used. Sample included 78 patients with untreated leprosy (G1), their 54 household contacts (G2), and 80 healthy individuals for the negative control (G3). The presence of M. leprae in both G1 and G2 was observed with the nasal swab and the palate mucosa scrapings methods, and it was shown that the sensitivity between the qPCR exams for RLEP and 85B genes is equivalent, with no statistically significant differences (G1 positivity of 35% in the hard palate mucosa and 44% for the nasal one, p = 0.3731 and for G2 of 31 and 38%, respectively, p = 0.6774). Results support the fact that the buccal mucosa and nasal mucosa may be important sites of primary infection of leprosy with repercussion in the transmission chain and that asymptomatic household contacts are heavily harbored by the causative agent of leprosy, which has a critical significance in the prevention and control action of this disease, since the evaluation of these sites arises as of importance in the early detection of M. leprae. Close monitoring and chemoprophylaxis of household contacts appear to be critical to attain interruption of the transmission of leprosy in endemic countries.
Similar content being viewed by others
References
Weekly epidemiological record Relevé épidémiologique hebdomadaire (2017) 1st September, 92th year, n° 35, 2017, 92, 501–520
Hastings RC (1994) Leprosy, 2nd edn. Churchill Livingstone, New York
Santos AR, Degrave WM, Suffys PN (1999) Use of polymerase chain reaction (PCR) in leprosy research. Indian J Lepr 71(1):101–110
Patrocínio LG, Goulart IM, Goulart LR, Patrocínio JA, Ferreira FR, Fleury RN (2005) Detection of Mycobacterium leprae in nasal mucosa biopsies by the polymerase chain reaction. FEMS Immunol Med Microbiol 44(3):311–316
Brasil. Ministério da Saúde (2013) Boletim Epidemiológico - Situação epidemiológica da hanseníase no Brasil – análise de indicadores selecionados na última década e desafios para eliminação. vol 44
Martinez TS, Figueira M, Costa A, Gonçalves M, Goulart L, Goulart I (2011) Oral mucosa as a source of Mycobacterium leprae infection and transmission, and implications of bacterial DNA detection and the immunological status. Clin Microbiol Infect 17(11):1653–1658
Martinez AN, Britto CF, Nery JA, Sampaio EP, Jardim MR, Sarno EN, Moraes MO (2006) Evaluation of real-time and conventional PCR targeting complex 85 genes for detection of Mycobacterium leprae DNA in skin biopsy samples from patients diagnosed with leprosy. J Clin Microbiol 44(9):3154–3159
Truman RW, Andrews PK, Robbins NY, Adams LB, Krahenbuhl JL et al (2008) Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl Trop Dis 2(11):e328
Motta ACF, Komesu MC, Silva CHL, Arruda D, Simão J, Zenha E, Furini RB, Foss NT (2008) Leprosy-specific oral lesions: a report of three cases. Med Oral Patol Oral Cir Bucal 13(8):479–482
Palaskar S (2004) Histopathological study of apparently normal oral mucosa in lepromatous leprosy. Indian J Dental Res 16(1):12–14
Girdhar B, Desikan K (1979) A clinical study of the mouth in untreated lepromatous patients. Lepr Rev 50(1):25
Davey TF, Rees RJ (1974) The nasal discharge in leprosy: clinical and bacteriological aspects. Lepr Rev 45(2):121–134
Desikan K (1977) Viability of Mycobacterium leprae outside the human body. Lepr Rev 48(4):231
da Costa APF, da Costa Nery JA, de Oliveira MLW-D, Cuzzi T, Ramos-e-Silva M (2003) Oral lesions in leprosy. Indian J Dermatol Venereol Leprol 69(6)
Sharma VK, Kaur S, Radotra BD, Kaur I (1993) Tongue involvement in lepromatous leprosy. Int J Dermatol 32(1):27–29
Brasil J, Opromolla D, Freitas J, Rossi J (1973) Histologic and bacteriologic study of lepromatous lesions of the oral mucosa. Estomatol Cult 7(2):113
Kumar B, Yande R, Kaur I, Mann S, Kaur S (1988) Involvement of palate and cheek in leprosy. Indian J Lepr 60(2):280–284
de Abreu MAMM, Michalany NS, Weckx LLM, Neto Pimentel DR, Hirata CHW, Alchorne MMA (2006) The oral mucosa in leprosy: a clinical and histopathological study. Rev Bras Otorrinolaringol 72(3):312–316
Cree I, Smith W (1998) Leprosy transmission and mucosal immunity: towards eradication? Lepr Rev 69(2):112–121
Pallagatti S, Sheikh S, Kaur A, Aggarwal A, Singh R (2012) Oral cavity and leprosy. Indian Dermatol Online J 3(2):101–104
Topazian RG, Goldberg MH, Hupp JR (2002) Oral and maxillofacial infections. 4ª edn. Elsevier Health Sciences,
Puy CL (2006) The role of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal 11:E449–E455
Martinez KO, Mendes LL, Alves JB (2007) Secretory A immunoglobulin, total proteins and salivary flow in recurrent aphthous ulceration. Rev Bras Otorrinolaringol 73(3):323–328
Van Beers SM, Izumi S, Madjid B, Maeda Y, Day R, Klatser PR (1994) An epidemiological study of leprosy infection by serology and polymerase chain reaction. Int J Lepr Other Mycobact Dis 62:1–9
Beyene D, Aseffa A, Harboe M, Kidane D, Macdonald M, Klatser P, Bjune G, Smith W (2003) Nasal carriage of Mycobacterium leprae DNA in healthy individuals in Lega Robi village, Ethiopia. Epidemiol Infect 131(02):841–848
Torres P, Camarena JJ, Gomez JR, Nogueira JM, Gimeno V, Navarro JC, Olmos A (2003) Comparison of PCR mediated amplification of DNA and the classical methods for detection of Mycobacterium leprae in different types of clinical samples in leprosy patients and contacts. Lepr Rev 74(1):18–30
Brand P (1959) Temperature variation and leprosy deformity. Int J Lepr 27(1):1–7
Scheepers A (1998) Correlation of oral surface temperatures and the lesions of leprosy. Int J Lepr Other Mycobact Dis 66:214–217
Scheepers A, Lemmer J, Lownie J (1993) Oral manifestations of leprosy. Lepr Rev 64(1):37–43
da Silva Martinez T, Nahas AA, Figueira MM, Costa AV, Goncalves MA, Goulart LR, Goulart IM (2011) Oral lesion in leprosy: borderline tuberculoid diagnosis based on detection of Mycobacterium leprae DNA by qPCR. Acta Derm Venereol 91(6):704–707. https://doi.org/10.2340/00015555-1175
Bucci F Jr, Mesa M, Schwartz R, McNeil G, Lambert W (1987) Oral lesions in lepromatous leprosy. J Oral Med 42(1):4
Rendall J, McDougall A, Willis L (1976) Intra-oral temperatures in man with special reference to involvement of the central incisors and premaxillary alveolar process in lepromatous leprosy. Int J Lepr Other Mycobact Dis 44(4):462–468
Martinez AN, Ribeiro-Alves M, Sarno EN, Moraes MO (2011) Evaluation of qPCR-based assays for leprosy diagnosis directly in clinical specimens. PLoS Negl Trop Dis 5(10):e1354
Goulart IMB, Goulart LR (2008) Leprosy: diagnostic and control challenges for a worldwide disease. Arch Dermatol Res 300(6):269–290
Funding
This study was partially funded by a grant from Fundação Paulista Contra a Hanseníase.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was submitted to the Ethical Committee on Research of the Instituto Lauro de Souza Lima and approved as per the protocol no. 157/11.
Informed consent
A written informed consent with detailed information on the study was required from participants before their inclusion in the study.
Rights and permissions
About this article
Cite this article
Carvalho, R.S., Foschiani, I.M., Costa, M.R.S.N. et al. Early detection of M. leprae by qPCR in untreated patients and their contacts: results for nasal swab and palate mucosa scraping. Eur J Clin Microbiol Infect Dis 37, 1863–1867 (2018). https://doi.org/10.1007/s10096-018-3320-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3320-9