Abstract
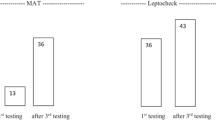
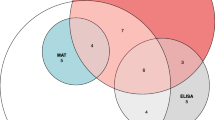
Leptospirosis and dengue are two commonly seen infectious diseases of the tropics. Differential diagnosis of leptospirosis from dengue fever is often difficult due to overlapping clinical symptoms and lack of economically viable and easy-to-perform laboratory tests. The gold standard for diagnosis is the microscopic agglutination test (MAT). In this study, the diagnostic potential of screening for pathogen-specific leptospiral antigens in urine samples is presented as a non-invasive method of disease diagnosis. In a study group of 40 patients, the serum was tested for anti-leptospiral antibodies by MAT and enzyme-linked immunosorbent assay (ELISA). Urine of these patients was screened for leptospiral antigens by ELISA using specific antibodies against LipL32, LipL41, Fla1, HbpA and sphingomyelinase. Group I patients (n = 23) were classified as leptospirosis-positive based on MAT and high titres of circulating IgM-specific anti-leptospiral antibodies. All of these patients excreted all five leptospiral antigens in the urine. The 17 MAT-negative cases included six patients with pyrexia of unknown origin (PUO; Group II) and 11 confirmed dengue patients (Group III). The latter tested negative for both serum anti-leptospiral antibodies and urinary leptospiral antigens. A salient outcome of this study was highlighting the usefulness of screening for urinary leptospiral antigens in disease diagnosis, as their presence confirmed leptospiral aetiology in two PUO patients. Immunoblots of urinary antigens identified well-defined bands corresponding to LipL32, HbpA and sphingomyelinase; the significance of the 42- and 58-kDa sphingomyelinase bands is discussed.




Similar content being viewed by others
References
Ko AI, Goarant C, Picardeau M (2009) Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7(10):736–747. https://doi.org/10.1038/nrmicro2208
Picardeau M (2015) Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis 9(9):e0004039. https://doi.org/10.1371/journal.pntd.0004039
Haake DA, Levett PN (2015) Leptospirosis in humans. Curr Top Microbiol Immunol 387:65–97. https://doi.org/10.1007/978-3-662-45059-8_5
Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM; Peru-United States Leptospirosis Consortium (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3(12):757–771
Sritharan M (2012) Insights into Leptospirosis, a neglected disease. In: Lorenzo-Morales J (ed) Zoonosis. InTech, Rijeka, Croatia, pp 167–192
Mishra B, Singhal L, Sethi S, Ratho RK (2013) Leptospirosis coexistent with dengue fever: a diagnostic dilemma. J Glob Infect Dis 5(3):121–122. https://doi.org/10.4103/0974-777X.116878
Zaki SA, Shanbag P (2010) Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection 38(4):285–291. https://doi.org/10.1007/s15010-010-0030-3
Kaur H, John M (2002) Mixed infection due to leptospira and dengue. Indian J Gastroenterol 21(5):206
Rele MC, Rasal A, Despande SD, Koppikar GV, Lahiri KR (2001) Mixed infection due to Leptospira and Dengue in a patient with pyrexia. Indian J Med Microbiol 19(4):206–207
Wijesinghe A, Gnanapragash N, Ranasinghe G, Ragunathan MK (2015) Fatal co-infection with leptospirosis and dengue in a Sri Lankan male. BMC Res Notes 8:348. https://doi.org/10.1186/s13104-015-1321-7
Levett PN (2001) Leptospirosis. Clin Microbiol Rev 14(2):296–326. https://doi.org/10.1128/CMR.14.2.296-326.2001
Aviat F, Rochereau-Roulet S, Branger C, Estavoyer JM, Chatrenet B, Orsonneau JL, Thorin C, Andre-Fontaine G (2010) Synthetic peptide issued from Hap1/LipL32 for new early serodiagnosis of human leptospirosis. Comp Immunol Microbiol Infect Dis 33(5):375–387. https://doi.org/10.1016/j.cimid.2009.01.001
Boonyod D, Poovorawan Y, Bhattarakosol P, Chirathaworn C (2005) LipL32, an outer membrane protein of Leptospira, as an antigen in a dipstick assay for diagnosis of leptospirosis. Asian Pac J Allergy Immunol 23(2-3):133–141
Fernandes CP, Seixas FK, Coutinho ML, Vasconcellos FA, Seyffert N, Croda J, McBride AJ, Ko AI, Dellagostin OA, Aleixo JA (2007) Monoclonal antibodies against LipL32, the major outer membrane protein of pathogenic Leptospira: production, characterization, and testing in diagnostic applications. Hybridoma (Larchmt) 26(1):35–41. https://doi.org/10.1089/hyb.2006.033
Tahiliani P, Kumar MM, Chandu D, Kumar A, Nagaraj C, Nandi D (2005) Gel purified LipL32: a prospective antigen for detection of leptospirosis. J Postgrad Med 51(3):164–168
Natarajaseenivasan K, Vijayachari P, Sharma S, Sugunan AP, Selvin J, Sehgal SC (2008) Serodiagnosis of severe leptospirosis: evaluation of ELISA based on the recombinant OmpL1 or LipL41 antigens of Leptospira interrogans serovar autumnalis. Ann Trop Med Parasitol 102(8):699–708. https://doi.org/10.1179/136485908X355229
Sarguna P, Rao MR, Sivakolundu S, Chaurasia R, Sritharan M, Shankar K (2015) Urban leptospirosis: a report of two cases. Indian J Med Microbiol 33(2):321–323. https://doi.org/10.4103/0255-0857.154898
Sivakolundu S, Sivakumar RR, Chidambaranathan GP, Sritharan M (2012) Serological diagnosis of leptospiral uveitis by HbpA IgG ELISA. J Med Microbiol 61(Pt 12):1681–1687. https://doi.org/10.1099/jmm.0.046870-0
Sridhar V, Manjulata Devi S, Ahmed N, Sritharan M (2008) Diagnostic potential of an iron-regulated hemin-binding protein HbpA that is widely conserved in Leptospira interrogans. Infect Genet Evol 8(6):772–776. https://doi.org/10.1016/j.meegid.2008.07.004
Alizadeh SA, Abdolahpour G, Pourmand MR, Naserpour T, Najafipour R, Eshraghi SS (2014) Evaluation of new ELISA based on rLsa63–rLipL32 antigens for serodiagnosis of human leptospirosis. Iran J Microbiol 6(3):184–189
Sankar S, Harshan HM, Somarajan SR, Srivastava SK (2010) Evaluation of a recombinant LigB protein of Leptospira interrogans serovar Canicola in an enzyme-linked immunosorbent assay for the serodiagnosis of bovine leptospirosis. Res Vet Sci 88(3):375–378. https://doi.org/10.1016/j.rvsc.2009.11.004
Fernando N, Wickremesinghe S, Niloofa R, Rodrigo C, Karunanayake L, de Silva HJ, Wickremesinghe AR, Premawansa S, Rajapakse S, Handunnetti SM (2016) Protein carbonyl as a biomarker of oxidative stress in severe Leptospirosis, and its usefulness in differentiating leptospirosis from dengue infections. PLoS One 11(6):e0156085. https://doi.org/10.1371/journal.pone.0156085
Conroy AL, Gélvez M, Hawkes M, Rajwans N, Liles WC, Villar-Centeno LA, Kain KC (2014) Host biomarkers distinguish dengue from leptospirosis in Colombia: a case–control study. BMC Infect Dis 14:35. https://doi.org/10.1186/1471-2334-14-35
Thresiamma KC, Bijou A, Chaurasia R, Sritharan M, Jayaprakash C, Thomas S, Eapen CK (2017) Proteinuria in early detection of human leptospirosis. Int J Res Med Sci 5(2):646–652
Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI (2007) Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 153(Pt 10):3390–3398. https://doi.org/10.1099/mic.0.2007/007948-0
Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, Davis J, Johnson M, Kuczek E, Alt DP, Peterson-Burch B, Coppel RL, Rood JI, Davies JK, Adler B (2006) Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A 103(39):14560–14565. https://doi.org/10.1073/pnas.0603979103
Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, Zhang YX, Xiong H, Lu G, Lu LF, Jiang HQ, Jia J, Tu YF, Jiang JX, Gu WY, Zhang YQ, Cai Z, Sheng HH, Yin HF, Zhang Y, Zhu GF, Wan M, Huang HL, Qian Z, Wang SY, Ma W, Yao ZJ, Shen Y, Qiang BQ, Xia QC, Guo XK, Danchin A, Saint Girons I, Somerville RL, Wen YM, Shi MH, Chen Z, Xu JG, Zhao GP (2003) Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422(6934):888–893. https://doi.org/10.1038/nature01597
Chou LF, Chen TW, Ko YC, Pan MJ, Tian YC, Chiu CH, Tang P, Hung CC, Yang CW (2014) Potential impact on kidney infection: a whole-genome analysis of Leptospira santarosai serovar Shermani. Emerg Microbes Infect 3(11):e82. https://doi.org/10.1038/emi.2014.78
Narayanavari SA, Sritharan M, Haake DA, Matsunaga J (2012) Multiple leptospiral sphingomyelinases (or are there?). Microbiology 158(Pt 5):1137–1146. https://doi.org/10.1099/mic.0.057737-0
Surujballi O, Mallory M (2004) An indirect enzyme linked immunosorbent assay for the detection of bovine antibodies to multiple Leptospira serovars. Can J Vet Res 68(1):1–6
Smythe LD, Wuthiekanun V, Chierakul W, Suputtamongkol Y, Tiengrim S, Dohnt MF, Symonds ML, Slack AT, Apiwattanaporn A, Chueasuwanchai S, Day NP, Peacock SJ (2009) The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovar in Thailand. Am J Trop Med Hyg 81(4):695–697. https://doi.org/10.4269/ajtmh.2009.09-0252
Llewellyn JR, Krupka-Dyachenko I, Rettinger AL, Dyachenko V, Stamm I, Kopp PA, Straubinger RK, Hartmann K (2016) Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from upper Bavaria. Berl Munch Tierarztl Wochenschr 129(5–6):251–257
Delaude A, Rodriguez-Campos S, Dreyfus A, Counotte MJ, Francey T, Schweighauser A, Lettry S, Schuller S (2017) Canine leptospirosis in Switzerland—a prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Prev Vet Med 141:48–60. https://doi.org/10.1016/j.prevetmed.2017.04.008
Costa F, Wunder EA Jr, De Oliveira D, Bisht V, Rodrigues G, Reis MG, Ko AI, Begon M, Childs JE (2015) Patterns in Leptospira shedding in Norway rats (Rattus norvegicus) from Brazilian slum communities at high risk of disease transmission. PLoS Negl Trop Dis 9(6):e0003819. https://doi.org/10.1371/journal.pntd.0003819
Murray GL (2013) The lipoprotein LipL32, an enigma of leptospiral biology. Vet Microbiol 162(2–4):305–314. https://doi.org/10.1016/j.vetmic.2012.11.005
Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA (2000) The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun 68(4):2276–2285
Malmström J, Beck M, Schmidt A, Lange V, Deutsch EW, Aebersold R (2009) Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature 460(7256):762–765. https://doi.org/10.1038/nature08184
Murray GL, Srikram A, Hoke DE, Wunder EA Jr, Henry R, Lo M, Zhang K, Sermswan RW, Ko AI, Adler B (2009) Major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans. Infect Immun 77(3):952–958. https://doi.org/10.1128/IAI.01370-08
Nally JE, Whitelegge JP, Bassilian S, Blanco DR, Lovett MA (2007) Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect Immun 75(2):766–773. https://doi.org/10.1128/IAI.00741-06
Monahan AM, Callanan JJ, Nally JE (2009) Review paper: host–pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol 46(5):792–799. https://doi.org/10.1354/vp.08-VP-0265-N-REV
Flannery B, Costa D, Carvalho FP, Guerreiro H, Matsunaga J, Da Silva ED, Ferreira AG, Riley LW, Reis MG, Haake DA, Ko AI (2001) Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J Clin Microbiol 39(9):3303–3310
Guerreiro H, Croda J, Flannery B, Mazel M, Matsunaga J, Galvão Reis M, Levett PN, Ko AI, Haake DA (2001) Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect Immun 69(8):4958–4968. https://doi.org/10.1128/IAI.69.8.4958-4968.2001
Alexander AD, Smith OH, Hiatt CW, Gleiser CA (1956) Presence of hemolysin in cultures of pathogenic leptospires. Proc Soc Exp Biol Med 91(2):205–211
Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, Creno S, Kuczek ES, Bommezzadri S, Davis JC, McGrath A, Johnson MJ, Boursaux-Eude C, Seemann T, Rouy Z, Coppel RL, Rood JI, Lajus A, Davies JK, Médigue C, Adler B (2008) Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3(2):e1607. https://doi.org/10.1371/journal.pone.0001607
Narayanavari SA, Kishore NM, Sritharan M (2012) Structural analysis of the Leptospiral sphingomyelinases: in silico and experimental evaluation of Sph2 as an Mg-dependent sphingomyelinase. J Mol Microbiol Biotechnol 22(1):24–34. https://doi.org/10.1159/000337013
Narayanavari SA, Lourdault K, Sritharan M, Haake DA, Matsunaga J (2015) Role of sph2 gene regulation in hemolytic and sphingomyelinase activities produced by Leptospira interrogans. PLoS Negl Trop Dis 9(8):e0003952. https://doi.org/10.1371/journal.pntd.0003952
Velineni S, Ramadevi S, Asuthkar S, Sritharan M (2009) Effect of iron deprivation on expression of sphingomyelinase in pathogenic serovar Lai. Online J Bioinform 10:241–258
Sritharan M (2006) Iron and bacterial virulence. Indian J Med Microbiol 24(3):163–164
Asuthkar S, Velineni S, Stadlmann J, Altmann F, Sritharan M (2007) Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect Immun 75(9):4582–4591. https://doi.org/10.1128/IAI.00324-07
Lin MH, Chang YC, Hsiao CD, Huang SH, Wang MS, Ko YC, Yang CW, Sun YJ (2013) LipL41, a hemin binding protein from Leptospira santarosai serovar Shermani. PLoS One 8(12):e83246. https://doi.org/10.1371/journal.pone.0083246
Acknowledgements
KCT, CKE, BJZ and RP would like to thank the management of MOSC Medical College and the Department of Biochemistry for facilitating this study. RC and MS acknowledge the University of Hyderabad for providing the basic infrastructural facilities.
Funding
The study was not funded by any funding agency. Routine maintenance funds given to MOSC Medical College Hospital (for clinical components) and University of Hyderabad (for experimental studies) were used for the study.
Author information
Authors and Affiliations
Contributions
MS conceptualised the study, designed the experiments and prepared the manuscript; RC performed the experiments and compiled the data; KCT coordinated the processing of the clinical samples and biochemical analyses; CKE, BJZ and RP were the clinical partners and coordinated patient selection, sample collection and collection of clinical data.
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflict of interest with reference to this article.
Ethical approval
Ethical clearance and informed consent from the study subjects were obtained from MOSC Medical College Hospital. Clearance from both the Institutional Ethical Committee and Institute Biosafety Committee from the University of Hyderabad were obtained for the study.
Informed consent
Informed consent from the study subjects were obtained as per the guidelines of the Ethical Clearance Committee of MOSC Medical College Hospital.
Rights and permissions
About this article
Cite this article
Chaurasia, R., Thresiamma, K.C., Eapen, C.K. et al. Pathogen-specific leptospiral proteins in urine of patients with febrile illness aids in differential diagnosis of leptospirosis from dengue. Eur J Clin Microbiol Infect Dis 37, 423–433 (2018). https://doi.org/10.1007/s10096-018-3187-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-3187-9