Abstract
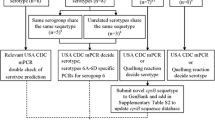
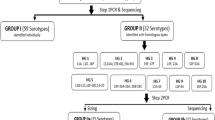
Serotyping of Streptococcus pneumoniae is essential for monitoring changes in the pneumococcal population and the impact of vaccines. Recently, various DNA-based methods have become available and are increasingly used because they are cheaper and easier to perform than the Quellung reaction. Our aim was to apply a DNA-based method, capsular sequence typing (CST), to a collection of non-viable lyophilized pneumococcal isolates dating from the 1980s to elucidate the serotypes circulating in Italy 30 years ago. As a preliminary evaluation of the method, CST was applied to 68 recent pneumococcal isolates representative of the most common serotypes circulating in Italy in invasive pneumococcal disease (IPD) previously serotyped by the Quellung reaction. CST was then applied to 132 lyophilized non-viable isolates. A serotype-specific polymerase chain reaction (PCR), using primers suggested by the Centers for Disease Control and Prevention (CDC), was performed when CST did not yield a univocal serotype. Considering the control isolates, CST concordance with the Quellung reaction was 95.6 %. For the non-viable lyophilized isolates, CST identified a univocal serotype for 59.4 % of the isolates. This percentage increased to 78.1 % if CST was combined with serotype-specific PCR. The most frequent serotypes in the collection of non-viable strains were: 3 (15.6 %), 14 (11.7 %), 35B (5.5 %), 19A (5.5 %), and 8 (4.7 %). CST proved to be a valid method for serotyping pneumococcal strains and provided information about pneumococcal serotypes present in Italy 30 years ago. The combination of CST with serotype-specific PCR was an effective strategy to identify pneumococcal serotypes that can be suggested also for routine laboratories.



Similar content being viewed by others
References
Bogaert D, De Groot R, Hermans PW (2004) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4(3):144–154. doi:10.1016/S1473-3099(04)00938-7
Kadioglu A, Weiser JN, Paton JC, Andrew PW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6(4):288–301. doi:10.1038/nrmicro1871
Grabenstein JD, Klugman KP (2012) A century of pneumococcal vaccination research in humans. Clin Microbiol Infect 18(Suppl 5):15–24. doi:10.1111/j.1469-0691.2012.03943.x
Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH (2015) Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev 28(3):871–899. doi:10.1128/CMR.00024-15
Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K (2000) Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 19(3):187–195
Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR; Active Bacterial Core Surveillance/Emerging Infections Program Network (2010) Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201(1):32–41. doi:10.1086/648593
Wyres KL, Lambertsen LM, Croucher NJ, McGee L, von Gottberg A, Liñares J, Jacobs MR, Kristinsson KG, Beall BW, Klugman KP, Parkhill J, Hakenbeck R, Bentley SD, Brueggemann AB (2013) Pneumococcal capsular switching: a historical perspective. J Infect Dis 207(3):439–449. doi:10.1093/infdis/jis703
Austrian R (1976) The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med 43(6):699–709
Lund E (1960) Laboratory diagnosis of Pneumococcus infections. Bull World Health Organ 23:5–13
Pai R, Gertz RE, Beall B (2006) Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 44(1):124–131. doi:10.1128/JCM.44.1.124-131.2006
Brito DA, Ramirez M, de Lencastre H (2003) Serotyping Streptococcus pneumoniae by multiplex PCR. J Clin Microbiol 41(6):2378–2384
Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV (2013) Evaluation of pneumococcal serotyping by multiplex PCR and quellung reactions. J Clin Microbiol 51(12):4193–4195. doi:10.1128/JCM.01876-13
Tarragó D, Fenoll A, Sánchez-Tatay D, Arroyo LA, Muñoz-Almagro C, Esteva C, Hausdorff WP, Casal J, Obando I (2008) Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin Microbiol Infect 14(9):828–834. doi:10.1111/j.1469-0691.2008.02028.x
Azzari C, Moriondo M, Indolfi G, Cortimiglia M, Canessa C, Becciolini L, Lippi F, de Martino M, Resti M (2010) Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS One 5(2):e9282. doi:10.1371/journal.pone.0009282
Elberse KE, van de Pol I, Witteveen S, van der Heide HG, Schot CS, van Dijk A, van der Ende A, Schouls LM (2011) Population structure of invasive Streptococcus pneumoniae in The Netherlands in the pre-vaccination era assessed by MLVA and capsular sequence typing. PLoS One 6(5):e20390. doi:10.1371/journal.pone.0020390
Bender MH, Yother J (2001) CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J Biol Chem 276(51):47966–47974. doi:10.1074/jbc.M105448200
Nielsen SV, Henrichsen J (1992) Capsular types of Streptococcus pneumoniae isolated from blood and CSF during 1982–1987. Clin Infect Dis 15(5):794–798
Mavroidi A, Godoy D, Aanensen DM, Robinson DA, Hollingshead SK, Spratt BG (2004) Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J Bacteriol 186(24):8181–8192. doi:10.1128/JB.186.24.8181-8192.2004
Elberse KE, van der Heide HG, Witteveen S, van de Pol I, Schot CS, van der Ende A, Berbers GA, Schouls LM (2012) Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine 30(52):7644–7651. doi:10.1016/j.vaccine.2012.04.021
Venkateswaran PS, Stanton N, Austrian R (1983) Type variation of strains of Streptococcus pneumoniae in capsular serogroup 15. J Infect Dis 147(6):1041–1054
Leung MH, Bryson K, Freystatter K, Pichon B, Edwards G, Charalambous BM, Gillespie SH (2012) Sequetyping: serotyping Streptococcus pneumoniae by a single PCR sequencing strategy. J Clin Microbiol 50(7):2419–2427. doi:10.1128/JCM.06384-11
Dube FS, van Mens SP, Robberts L, Wolter N, Nicol P, Mafofo J, Africa S, Zar HJ, Nicol MP (2015) Comparison of a real-time multiplex PCR and sequetyping assay for pneumococcal serotyping. PLoS One 10(9):e0137349. doi:10.1371/journal.pone.0137349
Elberse K, van Mens S, Cremers AJ, Meijvis SC, Vlaminckx B, de Jonge MI, Meis JF, Blauwendraat C, van de Pol I, Schouls LM (2015) Detection and serotyping of pneumococci in community acquired pneumonia patients without culture using blood and urine samples. BMC Infect Dis 15:56. doi:10.1186/s12879-015-0788-0
Jin P, Wu L, Oftadeh S, Kudinha T, Kong F, Zeng Q (2016) Using a practical molecular capsular serotype prediction strategy to investigate Streptococcus pneumoniae serotype distribution and antimicrobial resistance in Chinese local hospitalized children. BMC Pediatr 16(1):53. doi:10.1186/s12887-016-0589-7
De Bac C, Andreoni O, Fara GM, Giammanco G, La Placa M, Mascellino MT (1981) A multicentric study for serotyping and susceptibility to antibiotics of pneumococci in Italy. Ric Clin Lab 11(2):145–149
Luzzi I, Gianfrilli P, D’Angelantonio A (1981) Surveillance of Streptococcus pneumonia serotypes circulating in Italy. Ann Sclavo 23(5–6):562–568
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that ethical approval was not required.
Informed consent
The authors declare that informed consent was not required.
Additional information
G. Errico and C. Lucarelli contributed equally to this work.
Rights and permissions
About this article
Cite this article
Errico, G., Lucarelli, C., D’Ambrosio, F. et al. Application of capsular sequence typing (CST) to serotype non-viable Streptococcus pneumoniae isolates from an old collection. Eur J Clin Microbiol Infect Dis 35, 2025–2031 (2016). https://doi.org/10.1007/s10096-016-2755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2755-0