Abstract
Data on the occurrence and outcome of patients with chronic obstructive pulmonary disease (COPD) and ventilator-associated pneumonia (VAP) are quite limited. The aim of this study was to determine if COPD intensive care unit (ICU) patients have a higher rate of VAP development, different microbiological aetiology or have worse outcomes than other patients without VAP. A secondary analysis of a large prospective, observational study conducted in 27 European ICUs was carried out. Trauma patients were excluded. Of 2082 intubated patients included in the study, 397 (19.1 %) had COPD; 79 (19.9 %) patients with COPD and 332 (19.7 %) patients without COPD developed VAP. ICU mortality increased by 17 % (p < 0.05) when COPD patients developed VAP, remaining an independent predictor of mortality [odds ratio (OR) 2.28; 95 % confidence interval (CI) 1.35–3.87]. The development of VAP in COPD patients was associated with a median increase of 12 days in the duration of mechanical ventilation and >13 days in ICU stay (p < 0.05). Pseudomonas aeruginosa was more common in VAP when COPD was present (29.1 % vs. 18.7 %, p = 0.04) and was the most frequent isolate in COPD patients with early-onset VAP, with a frequency 2.5 times higher than in patients without early-onset VAP (33.3 % vs. 13.3 %, p = 0.03). COPD patients are not more predisposed to VAP than other ICU patients, but if COPD patients develop VAP, they have a worse outcome. Antibiotic coverage for non-fermenters needs to be included in the empiric therapy of all COPD patients, even in early-onset VAP.
Similar content being viewed by others
Introduction
Despite the availability of evidence-based guidelines and continuing innovations in prevention, ventilator-associated pneumonia (VAP) remains a significant threat for patients receiving prolonged mechanical ventilation in the intensive care unit (ICU) [1–3]. On average, 10–20 % of patients mechanically ventilated for more than 24 h develop VAP [4]. However, depending on the availability of resources and the specific risk profile of the index population, the overall prevalence may be substantially higher, reaching up to 40 % in particular patient subgroups [5, 6]. This high incidence is particularly significant given the important impact of VAP in terms of morbidity, mortality and healthcare costs [3, 7, 8]. The added morbidity of VAP contributes to an estimated excess length of hospitalisation of 4–6 days [4, 9]. Furthermore, patients with VAP may face a mortality risk twice that of similar ICU patients without VAP [4]. As with the risk of VAP onset, outcomes associated with VAP might vary according to the severity of acute illness and presence of underlying conditions [10, 11]. The clinical relevance of chronic respiratory disease to the occurrence and outcome of VAP warrants particular consideration.
Chronic obstructive pulmonary disease (COPD) is characterised by airflow limitation associated with an abnormal inflammatory response of the lungs to noxious gas particles [12–14]. In patients with advanced disease, emphysema may lead to further airflow limitation [12–14]. COPD is a major risk factor for community-acquired pneumonia (CAP), as the patient’s defences might be impaired due to long-term corticosteroid treatment and reduced microbial clearance [13–18]. Whether COPD increases mortality in CAP remains, however, controversial [19–24].
The relationship between COPD, VAP and mortality has not been thoroughly investigated. A previous single-centre study has reported increased ICU mortality associated with VAP in patients with COPD [25]. Using data from a large multicentre observational cohort, our objectives were to determine whether patients with COPD have a higher rate of development of VAP than those without COPD and whether patients with COPD who develop VAP have different microbiological aetiology and outcomes than patients without underlying COPD or COPD without VAP. Our hypothesis was that COPD patients who develop VAP might represent a unique patient group in terms of incidence, prognosis, aetiology and management.
Materials and methods
This study is a secondary analysis of the European (EU)-VAP/CAP database [26]. The EU-VAP/CAP project was a prospective, observational survey conducted in 27 ICUs from nine European countries: Belgium, France, Germany, Greece, Italy, Ireland, Portugal, Spain and Turkey. All patients requiring admission for a diagnosis of pneumonia or invasive mechanical ventilation for ≥48 h, irrespective of the admission diagnosis, were included. Patients that received only non-invasive mechanical ventilation were excluded. The target was the inclusion of 100 consecutive admissions in each ICU. This study was conducted in accordance with the 1964 declaration of Helsinki and its later amendments. The participating centres either received institutional ethical approval or had the requirement for ethical approval waived. Informed consent was waived due to the observational nature of the study and the collection of anonymised data.
Patient demographics, primary diagnosis, comorbidities, McCabe classification of comorbidities, Simplified Acute Physiology Score (SAPS) II on admission, ICU and hospital length of stay (LOS), duration of mechanical ventilation and ICU mortality were recorded for all patients. For patients with a clinical diagnosis of pneumonia as judged by the attending physicians, data collection included pneumonia type, clinical signs, sepsis severity (sepsis/severe sepsis/septic shock), SAPS II on the previous day and the day of clinical suspicion for VAP, Sequential Organ Failure Assessment (SOFA) score on the day of clinical suspicion of pneumonia, diagnostic procedures performed, microbiologic data, treatment data and outcome data [27–30]. The definition of septic shock followed published definitions from the 1992 American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference on sepsis and organ failure [27]. Organ dysfunction was defined according to SOFA score definitions [30].
Chronic disease status was categorised according to McCabe’s classification: non-fatal underlying disease or no underlying disease, ultimately fatal underlying disease (<5 years expected survival) and rapidly fatal underlying disease (<1 year expected survival) [27]. Each clinical episode of pneumonia was described separately. However, for patients who developed more than one episode of VAP, only variables related to the first episode of VAP were analysed.
VAP was defined as a pulmonary infection arising ≥48 h after endotracheal intubation with no evidence of pneumonia at the time of intubation or the diagnosis of a new pulmonary infection if the initial admission to ICU was for pneumonia [8]. Early-onset VAP was defined as VAP developing 2–4 days after intubation, while late-onset was defined as VAP developing ≥5 days after intubation. Microbiologically confirmed VAP (VAP with definite aetiology) was defined as VAP with a microorganism isolated from respiratory samples or blood in a patient with suspicion of pneumonia and characterised as definite aetiology of VAP according to clinical judgment and interpretation of specimen culture results [26]. COPD was defined as a pre-existing disease state characterised by the presence of airflow limitation due to chronic bronchitis or emphysema [19, 20]. The presence of COPD was recorded by the attending physician based on pulmonary function tests before ICU admission where available, or on clinical criteria, medical history (including significant smoking exposure, previous use of respiratory medications) and evidence of hyperinflation on chest radiograph [19, 20].
The study is reported in accordance with observational study guidelines [31]. Further information on the methods, variables and definitions of the study has been reported previously [26].
Trauma patients were excluded from the current analysis as they represent a group with distinct characteristics, including a significantly lower prevalence of COPD [32, 33]. Patients with and without COPD were compared. Also, patients with COPD that developed VAP were compared to those that did not develop VAP. Outcome variables in this analysis were length of ICU stay and duration of mechanical ventilation, mortality and time to event (ICU death) calculated from the date of VAP diagnosis.
Statistical analysis
VAP prevalence was compared between patients with and without COPD using Pearson’s Chi-squared test. VAP incidence was expressed as first episodes of VAP per 1000 ventilator days at risk across 26 out of 27 sites, given non-consecutive enrolment at one site. Incidence calculations were performed for mechanical ventilation days to VAP onset, with censoring at the cessation of mechanical ventilation in patients who did not develop VAP (mechanical ventilation days at risk). Categorical variables are expressed as frequency and percentage. Continuous variables are expressed as mean and standard deviation (SD) or median and inter-quartile range (IQR), depending on whether the data followed a normal or non-normal distribution, respectively. A Student’s t-test for normal data and a Mann–Whitney U-test or Kruskal–Wallis H-test for non-normal data were used to compare continuous variables between groups. A Pearson Chi-squared test or Fisher’s exact test was used to compare differences in categorical variables between groups. Logistic regression was used to identify independent predictors of ICU death in patients with COPD; variables identified in the bivariate analysis (p < 0.20; event rate >5 %) were included in the model; site was entered as an independent factor into the model to control for site differences in mortality. Odds ratios (ORs) and 95 % confidence intervals (CIs) are reported. Manual and backward stepwise techniques were used to identify the model with best fit. A two-sided p-value <0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS 21 (IBM Corporation, Armonk, New York).
Results
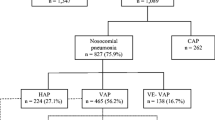
A total of 2082 patients were included in this analysis, of which 397 (19.1 %) had COPD; 79 COPD patients developed VAP (Fig. 1). Demographic and outcome characteristics of COPD patients compared to non-COPD patients are shown in Table 1. ICU mortality rates in COPD and non-COPD patients in our cohort were equivalent to predicted mortality based on SAPS II at ICU admission (the observed and predicted mortalities were 35 and 37 %, respectively).
The prevalence of VAP in 26 study centres with consecutive enrolment was 18.3 %; there was no significant difference between patients with or without COPD (18.6 % vs. 18.2 %, p = 0.89). The incidence of VAP was 18.2/1000 ventilator days at risk, with no significant difference between patients with or without COPD (16.7 vs. 18.6 per 1000 ventilator days at risk, p = 0.41). There was no statistically significant difference between the incidence of VAP in COPD and non-COPD patients when those with neurological major organ failure at ICU admission were excluded (15.5 vs. 17.5 per 1000 ventilation days at risk, p = 0.40) and when patients with CAP on admission were excluded (17.9 vs. 19.6 per 1000 ventilation days at risk, p = 0.53). The median onset of VAP was not significantly different between patients with and without COPD (6 [4–15] vs. 6 [4–10.8] days; p = 0.48).
There were no significant differences in the demographics, clinical characteristics, comorbidities and severity of illness at ICU admission (as assessed by SAPS II) between ICU patients with COPD who developed VAP and those who did not develop VAP (Table 2). VAP development was associated with a longer total duration of mechanical ventilation compared to those that did not develop VAP, including in the subgroup of survivors. Also, VAP development was associated with a longer ICU stay (13.5 days) compared to those without VAP, including among survivors (13 days). Patients with COPD and VAP had higher mortality than COPD patients without VAP (48.1 % vs. 31.1 %, p = 0.005). In patients with COPD, VAP (OR 2.28; 95 % CI 1.35–3.87) and SAPS II (OR 1.03; 95 % CI 1.02–1.05) were independent predictors of ICU mortality (Table 3). When patients with CAP on admission were excluded, VAP (OR 3.19; 95 % CI 1.56–6.51) and SAPS II (OR 1.06; 95 % CI 1.04–1.08) remained independent predictors of ICU mortality.
Physical and laboratory characteristics on VAP onset by COPD status in patients with VAP were similar, but with a higher prevalence of hypothermia and pleural effusion when COPD was present (data not shown). There were no significant differences in the frequency of septic shock at VAP onset between patients with and without COPD (38.4 % vs. 33.9 %, p = 0.47).
There was no difference in the prevalence of microbiologically confirmed VAP between patients with and without COPD (Table 4). The type of diagnostic technique used (bronchoscopic vs. non-bronchoscopic) did not affect the rate of microbiologic confirmation (74.7 % % vs. 76.5 %, p = 0.41).
In COPD patients, there was a higher prevalence of Pseudomonas aeruginosa VAP (overall and with early-onset) compared to those without COPD (Table 4). When patients admitted with CAP were excluded, the prevalence of P. aeruginosa VAP remained higher in patients with COPD than those without (26.4 % vs. 15.8 %, respectively; p = 0.037). Overall, the prevalence of non-fermenting Gram-negative bacilli was higher in COPD patients (p = 0.056), especially in those with early-onset VAP (54.1 % vs. 20.0 % in patients with and without COPD, respectively; p < 0.001). This difference between COPD and non-COPD patients remained significant when patients with CAP were excluded (49.9 % vs. 18.5 %, respectively; p = 0.020). There was no difference in the mean onset of P. aeruginosa VAP in patients with and without COPD (6 [3–16] vs. 8.5 [4.0–16.0] days, p = 0.38).
Discussion
This is the largest study examining the relationships between COPD and VAP from many different European centres. Although COPD was not associated with a higher incidence of VAP than in non-COPD patients, we found that VAP development in COPD patients was associated with a 17 % higher mortality than in those not developing VAP, as well as with increased duration of mechanical ventilation and length of ICU stay. In addition, COPD was associated with a higher prevalence of VAP due to non-fermenting Gram-negative bacilli, notably in early-onset episodes. This association has important potential implications for the selection of empirical antibiotic therapy.
The prevalence of VAP in our cohort falls within the wide range of 6–27 % previously reported [8]. This high variability appears attributable to differences in case-mix and definitions for VAP. For example, the reported VAP prevalence of 6 % was limited to microbiologically confirmed VAP with quantitative tracheal aspirates cultures ≥106 cfu/mL and high rates of prior antibiotic use [25]. Regarding COPD status, we found that VAP prevalence and incidence were not different in patients with and without COPD. This contradicts a number of earlier studies that identified COPD as an independent risk factor for VAP development with a reported OR or relative risk varying from 1.4 to 3.9 [34–36]. Similar to our findings, a large prospective observational study found no significant difference in the incidence of VAP between patients with non-exacerbated COPD and those without COPD (11.9 vs. 16.0 per 1000 mechanically ventilated days, p = 0.40) [37].
A single-centre case–control study that examined the impact of VAP on patients with COPD found that VAP was independently associated with ICU mortality (OR 7.7; 95 % CI 3.2–18.6) [25]. We also found that VAP development independently increased the risk of death of COPD patients (OR 2.21), confirming the results of the above study in a multicentre, multinational cohort. Our findings that VAP development in COPD patients increased the median duration of mechanical ventilation by 12 days and the median ICU stay by 13.5 days are also in line with the results of the above study that found a mean increase of 11 days in both the duration of mechanical ventilation and the length of ICU stay [25].
In relation to the microbiological flora causing VAP in COPD patients, we found that P. aeruginosa was more frequently isolated in VAP patients when COPD was present. It has been previously reported that P. aeruginosa (31 %) was the most frequently isolated pathogen in COPD patients with microbiologically confirmed VAP, and that the risk of VAP due to P. aeruginosa was increased in patients with COPD [25, 38, 39]. We found that P. aeruginosa was the second most common pathogen in COPD patients (29 %), behind Enterobacteriaceae. Of note, non-fermenting Gram-negative bacilli were isolated 2.5 times more often in COPD patients with early-onset VAP, with P. aeruginosa being the most frequent isolate (33.3 %). This observation has obvious implications in the selection of the appropriate empirical antibiotic regimen when VAP is suspected in COPD patients [8]. Chronic use of corticosteroids in COPD patients might be the reason for this opportunistic pathogen being more frequently observed as a cause of VAP. Unfortunately, our database did not allow checking for the relationship between corticosteroid exposure and P. aeruginosa. However, chronic steroid use was included in the definition of immunosuppression, and the prevalence of immunosuppression did not differ significantly between VAP patients with and without COPD.
The current study, to the best of our knowledge, is the first to explore in detail the relationship between COPD and VAP in a multicentre cohort and the first that describes differences in the aetiology of VAP by COPD status, separately in early- and late-onset VAP.
Whilst this is a ‘real-world clinical study, it does have several limitations. First, it was an observational study with centres predominantly in central and southern Europe. The non-random selection of sites may mean that the results, including the incidence and microbiology of pneumonia, may not be widely generalisable. Similarly, the prevalence and incidence of VAP cannot be extrapolated to all ICU patients because we focused on non-trauma patients requiring invasive mechanical ventilation. Second, this was a secondary analysis of a study not specifically designed to look at the association of COPD and VAP. Therefore, there may be unmeasured factors that modulate the risk and outcomes of VAP in patients with COPD. Finally, data on the severity of COPD as defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) were not recorded and, therefore, we could not explore the potential relationship between the severity of COPD and the occurrence of VAP or mortality [12, 14]. However, including only COPD patients with pulmonary function tests before ICU admission would lead to a selection bias and an underestimation of COPD incidence [40]. Not all patients with COPD are diagnosed before critical illness; on the contrary, it is not uncommon for COPD to be diagnosed for the first time during ICU admission [40]. The probability of COPD is very high in patients with smoking history, signs of hyperinflation and/or emphysema in chest X-ray and/or expiratory flow limitation during control mechanical ventilation [40]. In everyday clinical practice, it is common that ICU physicians need to make decisions regarding empirical antibiotic treatment for COPD patients with a suspicion of VAP without having available information on pulmonary function tests and GOLD classification.
Conclusions
Our findings have several important implications for clinical practice. First, they highlight the significant impact of ventilator-associated pneumonia (VAP) development on the morbidity and mortality of patients with chronic obstructive pulmonary disease (COPD) and the need for rigorous VAP prevention measures and prompt initiation of appropriate empirical treatment when VAP is suspected. Second, our findings are consistent with prior studies on colonisation or risk factors for Pseudomonas aeruginosa in mechanically ventilated patients. They suggest the inclusion of an anti-pseudomonal antibiotic in the initial empirical antibiotic treatment of VAP when COPD is present, regardless of the time of onset. Due to the risk of delaying inappropriate therapy, these findings should be considered in future guidelines. Very limited outcome data are available and the current study may act as a platform for a randomised clinical trial specifically designed to improve outcomes. This study reinforces the diversity of VAP, in contrast with community-acquired pneumonia (CAP), and the need to consider VAP as a heterogeneous entity influenced by patient case-mix.
References
Lorente L, Blot S, Rello J (2010) New issues and controversies in the prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med 182:870–876. doi:10.1164/rccm.201001-0081CI
Blot S, Rello J, Vogelaers D (2011) What is new in the prevention of ventilator-associated pneumonia? Curr Opin Pulm Med 17:155–159. doi:10.1097/MCP.0b013e328344db65
Nair GB, Niederman MS (2015) Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 41:34–48. doi:10.1007/s00134-014-3564-5
Safdar N, Dezfulian C, Collard HR, Saint S (2005) Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med 33:2184–2193
Kanafani ZA, Kara L, Hayek S, Kanj SS (2003) Ventilator-associated pneumonia at a tertiary-care center in a developing country: incidence, microbiology, and susceptibility patterns of isolated microorganisms. Infect Control Hosp Epidemiol 24:864–869. doi:10.1086/502151
Rodriguez JL, Gibbons KJ, Bitzer LG, Dechert RE, Steinberg SM, Flint LM (1991) Pneumonia: incidence, risk factors, and outcome in injured patients. J Trauma 31:907–912, discussion 912–4
Restrepo MI, Anzueto A, Arroliga AC et al (2010) Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect Control Hosp Epidemiol 31:509–515. doi:10.1086/651669
American Thoracic Society; Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi:10.1164/rccm.200405-644ST
Muscedere JG, Martin CM, Heyland DK (2008) The impact of ventilator-associated pneumonia on the Canadian health care system. J Crit Care 23:5–10. doi:10.1016/j.jcrc.2007.11.012
Martin-Loeches I, Deja M, Koulenti D et al (2013) Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med 39:672–681. doi:10.1007/s00134-012-2808-5
Myny D, Depuydt P, Colardyn F, Blot S (2005) Ventilator-associated pneumonia in a tertiary care ICU: analysis of risk factors for acquisition and mortality. Acta Clin Belg 60:114–121. doi:10.1179/acb.2005.022
Pauwels RA, Buist AS, Calverley PM et al (2001) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 163:1256–1276
Decramer M, Janssens W, Miravitlles M (2012) Chronic obstructive pulmonary disease. Lancet 379:1341–1351. doi:10.1016/S0140-6736(11)60968-9
(2015) From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed 28 Apr 2015
Crim C, Calverley PMA, Anderson JA et al (2009) Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 34:641–647. doi:10.1183/09031936.00193908
Ernst P, Gonzalez AV, Brassard P, Suissa S (2007) Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 176:162–166. doi:10.1164/rccm.200611-1630OC
Myles PR, McKeever TM, Pogson Z, Smith CJ, Hubbard RB (2009) The incidence of pneumonia using data from a computerized general practice database. Epidemiol Infect 137:709–716. doi:10.1017/S0950268808001428
Vinogradova Y, Hippisley-Cox J, Coupland C (2009) Identification of new risk factors for pneumonia: population-based case–control study. Br J Gen Pract 59:e329–e338. doi:10.3399/bjgp09X472629
Restrepo MI, Mortensen EM, Pugh JA, Anzueto A (2006) COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J 28:346–351. doi:10.1183/09031936.06.00131905
Rello J, Rodriguez A, Torres A et al (2006) Implications of COPD in patients admitted to the intensive care unit by community-acquired pneumonia. Eur Respir J 27:1210–1216. doi:10.1183/09031936.06.00139305
Molinos L, Clemente MG, Miranda B et al (2009) Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect 58:417–424. doi:10.1016/j.jinf.2009.03.003
Restrepo MI, Mortensen EM, Anzueto A (2011) Are COPD patients with pneumonia who are taking inhaled corticosteroids at higher risk of dying? Eur Respir J 38:1–3. doi:10.1183/09031936.00028711
Snijders D, van der Eerden M, de Graaff C, Boersma W (2010) The influence of COPD on mortality and severity scoring in community-acquired pneumonia. Respiration 79:46–53. doi:10.1159/000213757
Pifarre R, Falguera M, Vicente-de-Vera C, Nogues A (2007) Characteristics of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. Respir Med 101:2139–2144. doi:10.1016/j.rmed.2007.05.011
Nseir S, Di Pompeo C, Soubrier S et al (2005) Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest 128:1650–1656. doi:10.1378/chest.128.3.1650
Koulenti D, Lisboa T, Brun-Buisson C et al (2009) Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 37:2360–2368. doi:10.1097/CCM.0b013e3181a037ac
McCabe WR, Jackson GG (1962) Gram-negative bacteremia: I. Etiology and ecology. Arch Intern Med 110:847–855
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
[No authors listed] (1992) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577
Magret M, Amaya-Villar R, Garnacho J et al (2010) Ventilator-associated pneumonia in trauma patients is associated with lower mortality: results from EU-VAP study. J Trauma 69:849–854. doi:10.1097/TA.0b013e3181e4d7be
Cook A, Norwood S, Berne J (2010) Ventilator-associated pneumonia is more common and of less consequence in trauma patients compared with other critically ill patients. J Trauma 69:1083–1091. doi:10.1097/TA.0b013e3181f9fb51
Xie D-S, Xiong W, Lai R-P et al (2011) Ventilator-associated pneumonia in intensive care units in Hubei Province, China: a multicentre prospective cohort survey. J Hosp Infect 78:284–288. doi:10.1016/j.jhin.2011.03.009
Al-Dorzi HM, El-Saed A, Rishu AH et al (2012) The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control 40:794–799. doi:10.1016/j.ajic.2011.10.004
Tejerina E, Frutos-Vivar F, Restrepo MI et al (2006) Incidence, risk factors, and outcome of ventilator-associated pneumonia. J Crit Care 21:56–65. doi:10.1016/j.jcrc.2005.08.005
Rodríguez A, Lisboa T, Solé-Violán J et al (2011) Impact of nonexacerbated COPD on mortality in critically ill patients. Chest 139:1354–1360. doi:10.1378/chest.10-2439
Rello J, Ausina V, Ricart M et al (1994) Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med 20:193–198
Rello J, Borgatta B, Lisboa T (2013) Risk factors for Pseudomonas aeruginosa pneumonia in the early twenty-first century. Intensive Care Med 39:2204–2206. doi:10.1007/s00134-013-3046-1
Makris D, Desrousseaux B, Zakynthinos E, Durocher A, Nseir S (2011) The impact of COPD on ICU mortality in patients with ventilator-associated pneumonia. Respir Med 105(7):1022–1029
Acknowledgements
Authorship and contributorship
JR had full access to all of the data of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. DK and JR: study design, data analysis and interpretation, writing of the manuscript and final approval of the manuscript; SB: data collection, interpretation, writing of the manuscript and final approval of the manuscript; JMD: interpretation, writing of the manuscript and final approval of the manuscript; LP, IM-L, GD, CB-B, MN, CP, JS-V, AA: data collection, data interpretation, revision of the manuscript and final approval of the manuscript.
Conflict of interest
The authors have reported that no potential conflicts of interest exist related to the content of the article.
Collaborators: EU-VAP/CAP Study Group investigators
Djilali Annane (Raymond Poincaré University Hospital, Garches, France), Rosario Amaya-Villar (Virgen de Rocio University Hospital, Seville, Spain), Apostolos Armaganidis (Attikon University Hospital, Athens, Greece), Stijn Blot (1,2Ghent University Hospital, Ghent, Belgium, 1RBWH University of Queensland), Barbara Borgatta (1Vall d’Hebron University Hospital, Barcelona, Spain), Christian Brun-Buisson (Henri-Mondor University Hospital, Paris, France), Antonio Carneiro (2Santo Antonio Hospital, Porto, Portugal), Maria Deja (Charité University Hospital, Berlin, Germany), Jan DeWaele (Ghent University Hospital, Ghent, Belgium), Emili Diaz (Joan XIII University Hospital, Tarragona, Spain), George Dimopoulos (1,2Attikon University Hospital, 2Sotiria Hospital, Athens, Greece), Silvano Cardellino (Cardinal Massaia Hospital, Asti, Italy), Jose Garnacho-Montero (Virgen de Rocio University Hospital, Seville, Spain), Muhammet Guven (Erciyes University Hospital, Kayseri, Turkey), Apostolos Komnos (Larisa Hospital, Larisa, Greece), Despoina Koulenti (Study Coordinator, 1,2Attikon University Hospital, Athens, Greece, 1RBWH University of Queensland, 2Rovira i Virgili University, Tarragona, Spain), Wolfgang Krueger (1Constance Hospital, Constance, Germany, 2Tuebingen University Hospital, Tuebingen, Germany), Thiago Lisboa (2Joan XIII University Hospital, Tarragona, Spain and CIBER Enfermedades Respiratorias), Antonio Macor (Amedeo di Savoia Hospital, Torino, Italy), Emilpaolo Manno (Maria Vittoria Hospital, Torino, Italy), Rafael Mañez (Bellvitge University Hospital, Barcelona, Spain), Brian Marsh (Mater Misericordiae University Hospital, Dublin, Ireland), Claude Martin (Nord University Hospital, Marseille, France), Ignacio Martin-Loeches (1,2Mater Misericordiae University Hospital, Dublin, Ireland, and CIBERES, Spain), Pavlos Myrianthefs (KAT Hospital, Athens, Greece), Marc Nauwynck (St. Jan Hospital, Bruges, Belgium), Laurent Papazian (Hôpitaux de Marseille Aix-Marseille Université, 2Sainte-Marguerite University Hospital, Marseille, France), Christian Putensen (Bonn University Hospital, Bonn, Germany), Bernard Regnier (Claude Bernard University Hospital, Paris, France), Jordi Rello (Principal Investigator, 1Vall d’Hebron University Hospital, 2Joan XIII University Hospital, Tarragona, Spain and Vall d’Hebron University Hospital, CIBERES, Spain), Jordi Sole-Violan (Dr. Negrin University Hospital, Gran Canarias, Spain), Giuseppe Spina (Mauriziano Umberto I Hospital, Torino, Italy), Arzu Topeli (Hacettepe University Hospital, Ankara, Turkey), Hermann Wrigge (Bonn University Hospital, Bonn, Germany). [1Current affiliation, 2affiliation during the period of the study]
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
EU-VAP/CAP Study Group (collaborators): complete list in the Acknowledgements section.
The EU-VAP/CAP Study was endorsed by the European Critical Care Research Network (ECCRN) of the European Society of Intensive care Medicine (ESICM).
Rights and permissions
About this article
Cite this article
Koulenti, D., Blot, S., Dulhunty, J.M. et al. COPD patients with ventilator-associated pneumonia: implications for management. Eur J Clin Microbiol Infect Dis 34, 2403–2411 (2015). https://doi.org/10.1007/s10096-015-2495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2495-6