Abstract
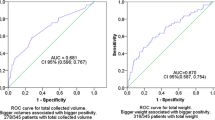
The yield of blood cultures is proportional to the volume of blood cultured. We evaluated an automatic blood volume monitoring system, recently developed by Becton Dickinson within its BACTEC EpiCenter module, that calculates mean volumes of negative aerobic bottles and generates boxplots and histograms. First, we evaluated the filling degree of 339 aerobic glass blood cultures by calculating the weight-based volume for each bottle. A substantial amount of the bottles (48.3 %) were inadequately filled. Evaluation of the accuracy of the monitoring system showed a mean bias of −1.4 mL (−15.4 %). Additional evaluation, using the amended software on 287 aerobic blood culture bottles, resulted in an acceptable mean deviation of −0.3 mL (−3.3 %). The new software version was also tested on 200 of the recently introduced plastic bottles, which will replace the glass bottles in the near future, showing a mean deviation of +2.8 mL (+26.7 %). In conclusion, the mean calculated volumes can be used for the training of a single phlebotomist. However, filling problems appear to be masked when using them for phlebotomist groups or on wards. Here, visual interpretation of boxplots and histograms can serve as a useful tool to observe the spread of the filling degrees and to develop a continuous improvement program. Re-adjustment of the software has proven to be necessary for use with plastic bottles. Due to our findings, BD has developed further adjustments to the software for validated use with plastic bottles, which will be released soon.



Similar content being viewed by others
References
Rodríguez-Créixems M, Alcalá L, Muñoz P, Cercenado E, Vicente T, Bouza E (2008) Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985–2006. Medicine (Baltimore) 87:234–249
Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K (2007) Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618
Suetens C (2008) Surveillance of nosocomial bloodstream infections in Belgian hospitals: results from the national surveillance network, 1992–2004 and 2007 (preliminary). Scientific Institute of Public Health, Brussels
York MK, Henry M, Gilligan P (2010) Section 3.4.1. Aerobic bacteriology, blood cultures, general detection and interpretation. In: Isenberg HD (ed) Clinical microbiology procedures handbook. American Society of Microbiology (ASM) Press, Washington, DC
Riedel S, Carroll KC (2010) Blood cultures: key elements for best practices and future directions. J Infect Chemother 16:301–316
Willems E, Smismans A, Cartuyvels R, Coppens G, Van Vaerenbergh K, Van den Abeele AM, Frans J; Bilulu Study Group (2012) The preanalytical optimization of blood cultures: a review and the clinical importance of benchmarking in 5 Belgian hospitals. Diagn Microbiol Infect Dis 73:1–8
Bouza E, Sousa D, Rodríguez-Créixems M, Lechuz JG, Muñoz P (2007) Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J Clin Microbiol 45:2765–2769
Cockerill FR 3rd, Wilson JW, Vetter EA, Goodman KM, Torgerson CA, Harmsen WS, Schleck CD, Ilstrup DM, Washington JA 2nd, Wilson WR (2004) Optimal testing parameters for blood cultures. Clin Infect Dis 38:1724–1730
Clinical and Laboratory Standards Institute (CLSI) (2007) Principles and procedures for blood cultures; Approved guideline. CLSI document M47-A. CLSI, Wayne, PA, pp 1–53
Cohen J, Brun-Buisson C, Torres A, Jorgensen J (2004) Diagnosis of infection in sepsis: an evidence-based review. Crit Care Med 32:S466–S494
Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE (2007) Reemergence of anaerobic bacteremia. Clin Infect Dis 44:895–900
Hecht DW (2006) Anaerobes: antibiotic resistance, clinical significance, and the role of susceptibility testing. Anaerobe 12:115–121
Bannister ER, Woods GL (1995) Evaluation of routine anaerobic blood cultures in the BacT/Alert blood culture system. Am J Clin Pathol 104:279–282
Horvath LL, George BJ, Hospenthal DR (2007) Detection of fifteen species of Candida in an automated blood culture system. J Clin Microbiol 45:3062–3064
Morris AJ, Wilson ML, Mirrett S, Reller LB (1993) Rationale for selective use of anaerobic blood cultures. J Clin Microbiol 31:2110–2113
Miller JM (1998) A guide to specimen management in clinical microbiology, 2nd edn. American Society of Microbiology (ASM) Press, Washington, DC
Thompson RB Jr, Miller JM (2007) Specimen collection, transport and processing: bacteriology. In: Murray PR, Baron EJ (eds) Manual of clinical microbiology, 9th edn. American Society of Microbiology (ASM) Press, Washington, DC, pp 291–310
James PA, Al-Shafi KM (2000) Clinical value of anaerobic blood culture: a retrospective analysis of positive patient episodes. J Clin Pathol 53:231–233
van Ingen J, Hilt N, Bosboom R (2013) Education of phlebotomy teams improves blood volume in blood culture bottles. J Clin Microbiol 51:1020–1021
Wilson ML, Harrell LJ, Mirrett S, Weinstein MP, Stratton CW, Reller LB (1992) Controlled evaluation of BACTEC PLUS 27 and Roche Septi-Chek anaerobic blood culture bottles. J Clin Microbiol 30:63–66
Weinstein MP, Mirrett S, Wilson ML, Reimer LG, Reller LB (1994) Controlled evaluation of 5 versus 10 milliliters of blood cultured in aerobic BacT/Alert blood culture bottles. J Clin Microbiol 32:2103–2106
Koontz FP, Flint KK, Reynolds JK, Allen SD (1991) Multicenter comparison of the high volume (10 ml) NR BACTEC PLUS system and the standard (5 ml) NR BACTEC system. Diagn Microbiol Infect Dis 14:111–118
Weinstein MP, Mirrett S, Reimer LG, Wilson ML, Smith-Elekes S, Chuard CR, Joho KL, Reller LB (1995) Controlled evaluation of BacT/Alert standard aerobic and FAN aerobic blood culture bottles for detection of bacteremia and fungemia. J Clin Microbiol 33:978–981
Mirrett S, Hanson KE, Reller LB (2007) Controlled clinical comparison of VersaTREK and BacT/ALERT blood culture systems. J Clin Microbiol 45:299–302
Reimer LG, Wilson ML, Weinstein MP (1997) Update on detection of bacteremia and fungemia. Clin Microbiol Rev 10:444–465
Jungkind DL, Thakur M, Dyke J (1989) Evidence for a second mechanism of action of resin in BACTEC NR 16A aerobic blood culture medium, abstr. C-225, p 430. In: Abstracts of the 89th Annual Meeting of the American Society for Microbiology 1989. American Society for Microbiology (ASM), Washington, DC
Acknowledgments
The authors would like to thank Cindy Germis, Liesbeth Van Acker, Caroline Pieters, and Line Coucke, coworkers in the microbiology lab of the AZ Sint-Lucas Hospital, for their outstanding technical assistance in the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coorevits, L., Van den Abeele, AM. Evaluation of the BD BACTEC FX blood volume monitoring system as a continuous quality improvement measure. Eur J Clin Microbiol Infect Dis 34, 1459–1466 (2015). https://doi.org/10.1007/s10096-015-2373-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2373-2