Abstract
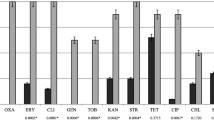
The purpose of this study was to investigate whether S. pseudintermedius is misdiagnosed as S. aureus by clinical laboratories when isolated from humans with dog bite wounds. In addition, we attempted to determine whether S. pseudintermedius isolates related to dog bite wounds share phenotypic and genotypic traits. S. pseudintermedius was identified by PCR targeting the nuc gene. Isolates were tested for antibiotic susceptibility using VetMIC GP-mo microdilution panels. The occurrence of genes encoding leukocidins, exfoliatins, pyrogenic toxin superantigens and enterotoxins was determined by PCR. The relatedness of S. pseudintermedius isolates was investigated using Multi Locus Sequence Typing (MLST). Out of 101 isolates defined as S. aureus by human clinical microbiology laboratories, 13 isolates were re-identified as S. pseudintermedius and one isolate was confirmed to carry the mecA gene, i.e. methicillin-resistant (MRSP). The MRSP isolate was also defined as multi-resistant. Two methicillin-susceptible S. pseudintermedius isolates were also multi-resistant and five were susceptible to all antibiotics tested. With the exception of three S. pseudintermedius isolates belonging to multi locus sequence type (MLST) 158, all the isolates belonged to unique STs. All isolates contained lukS/F-I, siet and se-int, and expA were identified in two isolates and expB and sec canine-sel in one isolate respectively. S. pseudintermedius is frequently misdiagnosed as S. aureus from humans with dog bite wounds showing that it can act as an opportunistic pathogen in humans. No common phenotypic and genotypic traits shared by the S. pseudintermedius isolates could be identified.
Similar content being viewed by others
References
Devriese LA, Hermans K, Baele M, Haesebrouck F (2009) Staphylococcus pseudintermedius versus Staphylococcus intermedius. Vet Microbiol 133(1–2):206–207
Perreten V, Kadlec K, Schwarz S, Gronlund Andersson U, Finn M, Greko C, Moodley A, Kania SA, Frank LA, Bemis DA, Franco A, Iurescia M, Battisti A, Duim B, Wagenaar JA, van Duijkeren E, Weese JS, Fitzgerald JR, Rossano A, Guardabassi L (2010) Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: an international multicentre study. J Antimicrob Chemother 65(6):1145–1154
Gharsa H, Ben Slama K, Gomez-Sanz E, Lozano C, Klibi N, Jouini A, Messadi L, Boudabous A, Torres C (2013) Antimicrobial resistance, virulence genes, and genetic lineages of Staphylococcus pseudintermedius in healthy dogs in tunisia. Microb Ecol 66(2):363–368
Gomez-Sanz E, Torres C, Benito D, Lozano C, Zarazaga M (2013) Animal and human Staphylococcus aureus associated clonal lineages and high rate of Staphylococcus pseudintermedius novel lineages in Spanish kennel dogs: predominance of S. aureus ST398. Vet Microbiol 166(3–4):580–589
Hanselman BA, Kruth SA, Rousseau J, Weese JS (2009) Coagulase positive staphylococcal colonization of humans and their household pets. Can Vet J 50(9):954–958
Paul NC, Bargman SC, Moodley A, Nielsen SS, Guardabassi L (2012) Staphylococcus pseudintermedius colonization patterns and strain diversity in healthy dogs: a cross-sectional and longitudinal study. Vet Microbiol 160(3–4):420–427
Morris DO, Rook KA, Shofer FS, Rankin SC (2006) Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003–04). Vet Dermatol 17(5):332–337
De Martino L, Lucido M, Mallardo K, Facello B, Mallardo M, Iovane G, Pagnini U, Tufano MA, Catalanotti P (2010) Methicillin-resistant staphylococci isolated from healthy horses and horse personnel in Italy. J Vet Diagn Invest 22(1):77–82
Himsworth CG, Patrick DM, Parsons K, Feng A, Weese JS (2013) Methicillin-resistant Staphylococcus pseudintermedius in rats. Emerg Infect Dis 19(1):169–170
Wettstein K, Descloux S, Rossano A, Perreten V (2008) Emergence of methicillin-resistant Staphylococcus pseudintermedius in Switzerland: three cases of urinary tract infections in cats. Schweiz Arch Tierheilkd 150(7):339–343
Kadlec K, Schwarz S, Perreten V, Andersson UG, Finn M, Greko C, Moodley A, Kania SA, Frank LA, Bemis DA, Franco A, Iurescia M, Battisti A, Duim B, Wagenaar JA, van Duijkeren E, Weese JS, Fitzgerald JR, Rossano A, Guardabassi L (2010) Molecular analysis of methicillin-resistant Staphylococcus pseudintermedius of feline origin from different European countries and North America. J Antimicrob Chemother 65(8):1826–1828
Ruscher C, Lubke-Becker A, Wleklinski CG, Soba A, Wieler LH, Walther B (2009) Prevalence of methicillin-resistant Staphylococcus pseudintermedius isolated from clinical samples of companion animals and equidaes. Vet Microbiol 136(1–2):197–201
Abrahamian FM, Goldstein EJ (2011) Microbiology of animal bite wound infections. Clin Microbiol Rev 24(2):231–246
Chuang CY, Yang YL, Hsueh PR, Lee PI (2010) Catheter-related bacteremia caused by Staphylococcus pseudintermedius refractory to antibiotic-lock therapy in a hemophilic child with dog exposure. J Clin Microbiol 48(4):1497–1498
Riegel P, Jesel-Morel L, Laventie B, Boisset S, Vandenesch F, Prevost G (2011) Coagulase-positive Staphylococcus pseudintermedius from animals causing human endocarditis. Int J Med Microbiol 301(3):237–239
Stegmann R, Burnens A, Maranta CA, Perreten V (2010) Human infection associated with methicillin-resistant Staphylococcus pseudintermedius ST71. J Antimicrob Chemother 65(9):2047–2048
Tanner MA, Everett CL, Youvan DC (2000) Molecular phylogenetic evidence for noninvasive zoonotic transmission of Staphylococcus intermedius from a canine pet to a human. J Clin Microbiol 38(4):1628–1631
Van Hoovels L, Vankeerberghen A, Boel A, Van Vaerenbergh K, De Beenhouwer H (2006) First case of Staphylococcus pseudintermedius infection in a human. J Clin Microbiol 44(12):4609–4612
Starlander G, Borjesson S, Gronlund-Andersson U, Tellgren-Roth C, Melhus A (2014) Cluster of infections caused by methicillin-resistant Staphylococcus pseudintermedius in a human hospital. J Clin Microbiol 52(8):3118–3120
Morris DO, Boston RC, O’Shea K, Rankin SC (2010) The prevalence of carriage of meticillin-resistant staphylococci by veterinary dermatology practice staff and their respective pets. Vet Dermatol 21(4):400–407
Talan DA, Staatz D, Staatz A, Overturf GD (1989) Frequency of Staphylococcus intermedius as human nasopharyngeal flora. J Clin Microbiol 27(10):2393
Devriese LA, Vancanneyt M, Baele M, Vaneechoutte M, De Graef E, Snauwaert C, Cleenwerck I, Dawyndt P, Swings J, Decostere A, Haesebrouck F (2005) Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int J Syst Evol Microbiol 55(Pt 4):1569–1573
Pottumarthy S, Schapiro JM, Prentice JL, Houze YB, Swanzy SR, Fang FC, Cookson BT (2004) Clinical isolates of Staphylococcus intermedius masquerading as methicillin-resistant Staphylococcus aureus. J Clin Microbiol 42(12):5881–5884
Clinical and Laboratory Standards Institute (CLSI) (2013) Performance standards for antimicrobial disk and dilution susceptiblity test for bacteria isolated from animals; Approved Standards - Fourth Edition, VET01-A4. CLSI, Wayne, PA
Nilsson P, Alexandersson H, Ripa T (2005) Use of broth enrichment and real-time PCR to exclude the presence of methicillin-resistant Staphylococcus aureus in clinical samples: a sensitive screening approach. Clin Microbiol Infect 11(12):1027–1034
Sasaki T, Tsubakishita S, Tanaka Y, Sakusabe A, Ohtsuka M, Hirotaki S, Kawakami T, Fukata T, Hiramatsu K (2010) Multiplex-PCR method for species identification of coagulase-positive staphylococci. J Clin Microbiol 48(3):765–769
Futagawa-Saito K, Makino S, Sunaga F, Kato Y, Sakurai-Komada N, Ba-Thein W, Fukuyasu T (2009) Identification of first exfoliative toxin in Staphylococcus pseudintermedius. FEMS Microbiol Lett 301(2):176–180
Futagawa-Saito K, Sugiyama T, Karube S, Sakurai N, Ba-Thein W, Fukuyasu T (2004) Prevalence and characterization of leukotoxin-producing Staphylococcus intermedius in isolates from dogs and pigeons. J Clin Microbiol 42(11):5324–5326
Iyori K, Hisatsune J, Kawakami T, Shibata S, Murayama N, Ide K, Nagata M, Fukata T, Iwasaki T, Oshima K, Hattori M, Sugai M, Nishifuji K (2010) Identification of a novel Staphylococcus pseudintermedius exfoliative toxin gene and its prevalence in isolates from canines with pyoderma and healthy dogs. FEMS Microbiol Lett 312(2):169–175
Lautz S, Kanbar T, Alber J, Lammler C, Weiss R, Prenger-Berninghoff E, Zschock M (2006) Dissemination of the gene encoding exfoliative toxin of Staphylococcus intermedius among strains isolated from dogs during routine microbiological diagnostics. J Vet Med B Infect Dis Vet Public Health 53(9):434–438
Hwang SY, Kim SH, Jang EJ, Kwon NH, Park YK, Koo HC, Jung WK, Kim JM, Park YH (2007) Novel multiplex PCR for the detection of the Staphylococcus aureus superantigen and its application to raw meat isolates in Korea. Int J Food Microbiol 117(1):99–105
Edwards VM, Deringer JR, Callantine SD, Deobald CF, Berger PH, Kapur V, Stauffacher CV, Bohach GA (1997) Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates. Infect Immun 65(6):2346–2352
Fitzgerald JR (2013) Evolution of Staphylococcus aureus during human colonization and infection. Infect Genet Evol 21:542–547
Gomez-Sanz E, Torres C, Lozano C, Saenz Y, Zarazaga M (2011) Detection and characterization of methicillin-resistant Staphylococcus pseudintermedius in healthy dogs in La Rioja, Spain. Comp Immunol Microbiol Infect Dis 34(5):447–453
Gomez-Sanz E, Torres C, Lozano C, Zarazaga M (2013) High diversity of Staphylococcus aureus and Staphylococcus pseudintermedius lineages and toxigenic traits in healthy pet-owning household members. Underestimating normal household contact? Comp Immunol Microbiol Infect Dis 36(1):83–94
Tanabe T, Toyoguchi M, Hirano F, Chiba M, Onuma K, Sato H (2013) Prevalence of staphylococcal enterotoxins in Staphylococcus pseudintermedius isolates from dogs with pyoderma and healthy dogs. Microbiol Immunol 57(9):651–654
Iyori K, Futagawa-Saito K, Hisatsune J, Yamamoto M, Sekiguchi M, Ide K, Son WG, Olivry T, Sugai M, Fukuyasu T, Iwasaki T, Nishifuji K (2011) Staphylococcus pseudintermedius exfoliative toxin EXI selectively digests canine desmoglein 1 and causes subcorneal clefts in canine epidermis. Vet Dermatol 22(4):319–326
Acknowledgments
The authors would like to thank the clinical microbiology laboratories for agreeing to include isolates in the study. A special thanks to all the laboratory technicians who helped with the submission of isolates to SVA.
The study was financed by Agria Animal Insurance Company and the Swedish Kennel Club’s joint research fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Börjesson, S., Gómez-Sanz, E., Ekström, K. et al. Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur J Clin Microbiol Infect Dis 34, 839–844 (2015). https://doi.org/10.1007/s10096-014-2300-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2300-y