Abstract
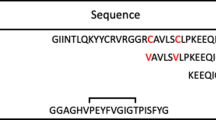
Tuberculosis (TB) is an ongoing threat to global health, and the lack of effective therapies for treating it is also a global problem. Previous studies have shown that human cathelicidin and defensins have effective antimicrobial activity against Mycobacterium spp. To our knowledge, there are no reports on the antimycobacterial effects of bovine neutrophil β-defensins so far. Here, we identified the antimicrobial effect of mature bovine neutrophil β-defensins (mBNBD) 5 against Mycobacterium infection both in vitro and in vivo. The mBNBD5 protein was expressed in Pichia pastoris. To increase the yield of β-defensins, a purification method was employed by adding a 6-His·tag to the C-terminus of the mBNBD5 gene. Our results indicated that recombinant mBNBD5 protein was successfully expressed and purified from Pichia pastoris with intact antimicrobial activity. The recombinant protein exhibited potent bactericidal activity in vitro against M. smegmatis and M. bovis, with a dose-dependent manner and a time-dependent manner. The electron microscope results showed that the bacterial cell wall of M. bovis was disrupted when incubated with mBNBD5 for 72 h. Our data also indicated that the exogenous addition of mBNBD5 could reduce the survival of Mycobacterium spp., especially M. tuberculosis and M. bovis in RAW 264.7 macrophages. These results provide foundations for the development of mBNBD5 as a potential new therapeutic agent for TB treatment.






Similar content being viewed by others
References
O’Reilly LM, Daborn CJ (1995) The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis 76(Suppl 1):1–46
Grange JM (2001) Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 81(1–2):71–77. doi:10.1054/tube.2000.0263
de la Rua-Domenech R (2006) Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb) 86(2):77–109. doi:10.1016/j.tube.2005.05.002
Thoen C, Lobue P, de Kantor I (2006) The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 112(2–4):339–345. doi:10.1016/j.vetmic.2005.11.047
[No authors listed] (1994) Zoonotic tuberculosis (Mycobacterium bovis): memorandum from a WHO meeting (with the participation of FAO). Bull World Health Organ 72(6):851–857
Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJ, Parsons SD, van Helden PD, Zinsstag J (2013) Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis 19(6):899–908. doi:10.3201/eid1906.120543
Cleaveland S, Shaw DJ, Mfinanga SG, Shirima G, Kazwala RR, Eblate E, Sharp M (2007) Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb) 87(1):30–43. doi:10.1016/j.tube.2006.03.001
Pinsky BA, Banaei N (2008) Multiplex real-time PCR assay for rapid identification of Mycobacterium tuberculosis complex members to the species level. J Clin Microbiol 46(7):2241–2246. doi:10.1128/JCM.00347-08
Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de Kantor I, Meslin FX (1998) Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis 4(1):59–70
Shah NP, Singhal A, Jain A, Kumar P, Uppal SS, Srivatsava MV, Prasad HK (2006) Occurrence of overlooked zoonotic tuberculosis: detection of Mycobacterium bovis in human cerebrospinal fluid. J Clin Microbiol 44(4):1352–1358. doi:10.1128/JCM.44.4.1352-1358.2006
Hancock RE, Nijnik A, Philpott DJ (2012) Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 10(4):243–254. doi:10.1038/nrmicro2745
Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4(7):529–536. doi:10.1038/nrmicro1441
Marr AK, Gooderham WJ, Hancock RE (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6(5):468–472. doi:10.1016/j.coph.2006.04.006
Fjell CD, Hiss JA, Hancock RE, Schneider G (2011) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11(1):37–51. doi:10.1038/nrd3591
Ganz T, Selsted ME, Szklarek D, Harwig SSL, Daher K, Bainton DF, Lehrer RI (1985) Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 76(4):1427–1435
Jarczak J, Kościuczuk EM, Lisowski P, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E (2013) Defensins: natural component of human innate immunity. Hum Immunol 74(9):1069–1079. doi:10.1016/j.humimm.2013.05.008
Menendez A, Brett Finlay B (2007) Defensins in the immunology of bacterial infections. Curr Opin Immunol 19(4):385–391. doi:10.1016/j.coi.2007.06.008
Méndez-Samperio P, Alba L, Trejo A (2007) Mycobacterium bovis-mediated induction of human beta-defensin-2 in epithelial cells is controlled by intracellular calcium and p38MAPK. J Infect 54(5):469–474. doi:10.1016/j.jinf.2006.08.009
Raj PA, Dentino AR (2002) Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett 206(1):9–18
Rivas-Santiago B, Schwander SK, Sarabia C, Diamond G, Klein-Patel ME, Hernandez-Pando R, Ellner JJ, Sada E (2005) Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 73(8):4505–4511. doi:10.1128/IAI.73.8.4505-4511.2005
Selsted ME, Tang YQ, Morris WL, McGuire PA, Novotny MJ, Smith W, Henschen AH, Cullor JS (1993) Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem 268(9):6641–6648
Ogata K, Linzer BA, Zuberi RI, Ganz T, Lehrer RI, Catanzaro A (1992) Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium–Mycobacterium intracellulare. Infect Immun 60(11):4720–4725
Ryan LK, Rhodes J, Bhat M, Diamond G (1998) Expression of beta-defensin genes in bovine alveolar macrophages. Infect Immun 66(2):878–881
Méndez-Samperio P (2008) Role of antimicrobial peptides in host defense against mycobacterial infections. Peptides 29(10):1836–1841. doi:10.1016/j.peptides.2008.05.024
Sonawane A, Santos JC, Mishra BB, Jena P, Progida C, Sorensen OE, Gallo R, Appelberg R, Griffiths G (2011) Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol 13(10):1601–1617. doi:10.1111/j.1462-5822.2011.01644.x
Rivas-Santiago B, Castañeda-Delgado JE, Rivas Santiago CE, Waldbrook M, González-Curiel I, León-Contreras JC, Enciso-Moreno JA, del Villar V, Mendez-Ramos J, Hancock REW, Hernandez-Pando R (2013) Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PLoS One 8(3):e59119. doi:10.1371/journal.pone.0059119
Ganz T, Lehrer RI (1998) Antimicrobial peptides of vertebrates. Curr Opin Immunol 10(1):41–44
Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL (1991) Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A 88(9):3952–3956
Cabral KM, Almeida MS, Valente AP, Almeida FC, Kurtenbach E (2003) Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: overcoming the inefficiency of the STE13 protease. Protein Expr Purif 31(1):115–122. doi:10.1016/s1046-5928(03)00136-0
Zhao P, Cao G (2012) Production of bioactive sheep beta-defensin-1 in Pichia pastoris. J Ind Microbiol Biotechnol 39(1):11–17. doi:10.1007/s10295-011-0992-x
Shi X, Karkut T, Chamankhah M, Alting-Mees M, Hemmingsen SM, Hegedus D (2003) Optimal conditions for the expression of a single-chain antibody (scFv) gene in Pichia pastoris. Protein Expr Purif 28(2):321–330. doi:10.1016/s1046-5928(02)00706-4
Huang Y, Zhou X, Bai Y, Yang L, Yin X, Wang Z, Zhao D (2012) Phagolysosome maturation of macrophages was reduced by PE_PGRS 62 protein expressing in Mycobacterium smegmatis and induced in IFN-gamma priming. Vet Microbiol 160(1–2):117–125. doi:10.1016/j.vetmic.2012.05.011
Schägger H (2006) Tricine-SDS-PAGE. Nat Protoc 1(1):16–22. doi:10.1038/nprot.2006.4
Mohammadzadeh T, Sadjjadi S, Habibi P, Sarkari B (2012) Comparison of agar dilution, broth dilution, cylinder plate and disk diffusion methods for evaluation of anti-leishmanial drugs on leishmania promastigotes. Iran J Parasitol 7(3):43–47
Zeya HI, Spitznagel JK (1968) Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med 127(5):927–941
Ganz T, Lehrer RI (1995) Defensins. Pharmacol Ther 66(2):191–205
Harder J, Bartels J, Christophers E, Schroder JM (2001) Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276(8):5707–5713. doi:10.1074/jbc.M008557200
Si L-G, Liu X-C, Lu Y-Y, Wang G-Y, Li W-M (2007) Soluble expression of active human β-defensin-3 in Escherichia coli and its effects on the growth of host cells. Chin Med J (Engl) 120(8):708–713
Li Y (2011) Recombinant production of antimicrobial peptides in Escherichia coli: a review. Protein Expr Purif 80(2):260–267. doi:10.1016/j.pep.2011.08.001
Bommarius B, Jenssen H, Elliott M, Kindrachuk J, Pasupuleti M, Gieren H, Jaeger KE, Hancock RE, Kalman D (2010) Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides 31(11):1957–1965. doi:10.1016/j.peptides.2010.08.008
Zorko M, Japelj B, Hafner-Bratkovic I, Jerala R (2009) Expression, purification and structural studies of a short antimicrobial peptide. Biochim Biophys Acta 1788(2):314–323. doi:10.1016/j.bbamem.2008.10.015
Corrales-Garcia L, Ortiz E, Castañeda-Delgado J, Rivas-Santiago B, Corzo G (2013) Bacterial expression and antibiotic activities of recombinant variants of human beta-defensins on pathogenic bacteria and M. tuberculosis. Protein Expr Purif 89(1):33–43. doi:10.1016/j.pep.2013.02.007
Kisich KO, Heifets L, Higgins M, Diamond G (2001) Antimycobacterial agent based on mRNA encoding human beta-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect Immun 69(4):2692–2699. doi:10.1128/IAI.69.4.2692-2699.2001
Nickel D, Busch M, Mayer D, Hagemann B, Knoll V, Stenger S (2012) Hypoxia triggers the expression of human beta defensin 2 and antimicrobial activity against Mycobacterium tuberculosis in human macrophages. J Immunol 188(8):4001–4007. doi:10.4049/jimmunol.1100976
Gonzalez-Curiel I, Castañeda-Delgado J, Lopez-Lopez N, Araujo Z, Hernandez-Pando R, Gandara-Jasso B, Macias-Segura N, Enciso-Moreno A, Rivas-Santiago B (2011) Differential expression of antimicrobial peptides in active and latent tuberculosis and its relationship with diabetes mellitus. Hum Immunol 72(8):656–662. doi:10.1016/j.humimm.2011.03.027
Rivas-Santiago B, Cervantes-Villagrana A, Sada E, Hernández-Pando R (2012) Expression of beta defensin 2 in experimental pulmonary tuberculosis: tentative approach for vaccine development. Arch Med Res 43(4):324–328. doi:10.1016/j.arcmed.2012.06.005
Rivas-Santiago CE, Rivas-Santiago B, León DA, Castañeda-Delgado J, Hernández Pando R (2011) Induction of beta-defensins by l-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin Exp Immunol 164(1):80–89. doi:10.1111/j.1365-2249.2010.04313.x
Kisich KO, Higgins M, Diamond G, Heifets L (2002) Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun 70(8):4591–4599. doi:10.1128/IAI.70.8.4591-4599.2002
Liu PT, Stenger S, Tang DH, Modlin RL (2007) Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179(4):2060–2063
Yount NY, Yeaman MR (2005) Immunocontinuum: perspectives in antimicrobial peptide mechanisms of action and resistance. Protein Pept Lett 12(1):49–67
Yount NY, Bayer AS, Xiong YQ, Yeaman MR (2006) Advances in antimicrobial peptide immunobiology. Biopolymers 84(5):435–458. doi:10.1002/bip.20543
Sharma S, Verma I, Khuller GK (1999) Biochemical interaction of human neutrophil peptide-1 with Mycobacterium tuberculosis H37Ra. Arch Microbiol 171(5):338–342
Anes E, Kühnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G (2003) Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol 5(9):793–802. doi:10.1038/ncb1036
Mittal M, Chakravarti S, Sharma V, Sanjeeth BS, Churamani CP, Kanwar NS (2014) Evidence of presence of Mycobacterium tuberculosis in bovine tissue samples by multiplex PCR: possible relevance to reverse zoonosis. Transbound Emerg Dis 61(2):97–104. doi:10.1111/tbed.12203
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, Project No. 2012AA101302), MoSTRCUK international cooperation project (Project No. 2013DFG32500), Chinese Universities Scientific Fund (Project No. 2013QT004), and CAU Foreign Experts Major Projects (Project No: 2013z018). We would also like to thank China Agricultural University for providing access to the BSL-3 Laboratories for experiments using M. bovis culture and infection.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jingjing Kang and Deming Zhao contributed to this work equally.
Rights and permissions
About this article
Cite this article
Kang, J., Zhao, D., Lyu, Y. et al. Antimycobacterial activity of Pichia pastoris-derived mature bovine neutrophil β-defensins 5. Eur J Clin Microbiol Infect Dis 33, 1823–1834 (2014). https://doi.org/10.1007/s10096-014-2152-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2152-5