Abstract
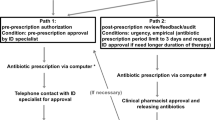
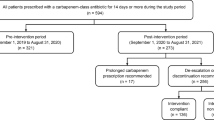
The objective of this study was to assess the impact on carbapenems use of a program combining pre-authorization requirement and systematic post-prescription review of carbapenems prescriptions. The program was implemented in a 1,230-bed teaching tertiary hospital. Monthly carbapenems consumption was analyzed using a controlled interrupted time-series method and compared to that of vancomycin before and after implementation of the intervention. Compared to the pre-intervention period (14 monthly points), a significant and sustained decrease of carbapenems consumption [1.66 defined daily doses (DDD)/1,000 patient-days; p = 0.048] was observed during the intervention period (12 monthly points), despite an increasing trend in incidence of extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) isolates (0.02/1,000 patient-days per month; p = 0.093). As expected, vancomycin consumption was unaffected by the intervention. A total of 337 prescriptions were reviewed in the intervention period; most were microbiologically documented (81.3 %; ESBL-PE: 39.2 %). Three of four (76.6 %) carbapenems prescriptions were modified within a median [interquartile range] of 2 [1; 4] days, either after infectious disease physician (IDP) advice (48.4 %) or by ward physicians (28.2 %). Most changes included de-escalating (52.2 %) or reducing the planned duration (22.2 %), which resulted in a median duration of treatment of only 3 [2; 7] days. The median length of stay and mortality rate were not influenced by the intervention. This reasonably practicable antimicrobial stewardship program including controlled delivery and systematic reevaluation of carbapenems prescriptions was able to reduce their use in our hospital, despite a rising ESBL-PE incidence.


Similar content being viewed by others
References
Pfaller MA, Segreti J (2006) Overview of the epidemiological profile and laboratory detection of extended-spectrum beta-lactamases. Clin Infect Dis 42:S153–S163
Livermore DM, Canton R, Gniadkowski M et al (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174
Hilty M, Betsch BY, Bögli-Stuber K et al (2012) Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis 55:967–975
Turner PJ (2005) Extended-spectrum beta-lactamases. Clin Infect Dis 41:S273–S275
Paterson DL, Bonomo RA (2005) Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18:657–686
Vardakas KZ, Tansarli GS, Rafailidis PI et al (2012) Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803
Cantón R, Akóva M, Carmeli Y et al (2012) Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431
Vaux S, Carbonne A, Thiolet JM et al (2011) Emergence of carbapenemase-producing Enterobacteriaceae in France, 2004 to 2011. Euro Surveill 16. pii: 19880
Bratu S, Landman D, Haag R et al (2005) Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 165:1430–1435
Giakoupi P, Maltezou H, Polemis M et al (2009) KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill 14. pii: 19218
Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S et al (2008) Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028–1033
Gasink LB, Edelstein PH, Lautenbach E et al (2009) Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol 30:1180–1185
Mouloudi E, Protonotariou E, Zagorianou A et al (2010) Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 31:1250–1256
Ramsay C, Brown E, Hartman G et al (2003) Room for improvement: a systematic review of the quality of evaluations of interventions to improve hospital antibiotic prescribing. J Antimicrob Chemother 52:764–771
Ramsay CR, Matowe L, Grilli R et al (2003) Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 19:613–623
Davey P, Brown E, Fenelon L et al (2005) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 4:CD003543
Lesprit P, Duong T, Girou E et al (2009) Impact of a computer-generated alert system prompting review of antibiotic use in hospitals. J Antimicrob Chemother 63:1058–1063
Gauzit R, Gutmann L, Brun-Buisson C et al (2010) Guidelines for good practice of carbapenems. Antibiotiques 12:183–189
Dellit TH, Owens RC, McGowan JE et al (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Lesprit P, Landelle C, Girou E et al (2010) Reassessment of intravenous antibiotic therapy using a reminder or direct counselling. J Antimicrob Chemother 65:789–795
Solomon DH, Van Houten L, Glynn RJ et al (2001) Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med 161:1897–1902
Elligsen M, Walker SA, Pinto R et al (2012) Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 33:354–361
Lesprit P, Landelle C, Brun-Buisson C (2013) Unsolicited post-prescription antibiotic review in surgical and medical wards: factors associated with counselling and physicians’ compliance. Eur J Clin Microbiol Infect Dis 32:227–235
Lepper PM, Grusa E, Reichl H et al (2002) Consumption of imipenem correlates with beta-lactam resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 46:2920–2925
Livermore DM, Hope R, Mushtaq S et al (2008) Orthodox and unorthodox clavulanate combinations against extended-spectrum β-lactamase producers. Clin Microbiol Infect 14:189–193
Rodríguez-Baño J, Navarro MD, Retamar P et al (2012) β-lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174
Rodríguez-Baño J, Picón E, Navarro MD et al (2012) Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli. Clin Microbiol Infect 18:894–900
Peterson LR (2008) Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin–tazobactam. Clin Microbiol Infect 14:181–184
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delory, T., De Pontfarcy, A., Emirian, A. et al. Impact of a program combining pre-authorization requirement and post-prescription review of carbapenems: an interrupted time-series analysis. Eur J Clin Microbiol Infect Dis 32, 1599–1604 (2013). https://doi.org/10.1007/s10096-013-1918-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1918-5