Abstract
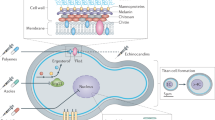
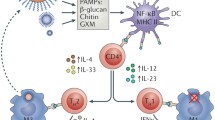
Cryptococcosis is an important systemic mycosis and the third most prevalent disease in human immunodeficiency virus (HIV)-positive individuals. The incidence of cryptococcosis is high among the 25 million people with HIV/acquired immunodeficiency syndrome (AIDS), with recent estimates indicating that there are one million cases of cryptococcal meningitis globally per year in AIDS patients. In Cryptococcus neoformans, resistance to azoles may be associated with alterations in the target enzyme encoded by the gene ERG11, lanosterol 14α-demethylase. These alterations are obtained through mutations, or by overexpressing the gene encoding. In addition, C. gattii and C. neoformans present a heteroresistance phenotype, which may be related to increased virulence. Other species beyond C. neoformans and C. gattii, such as C. laurentii, have been diagnosed mainly in patients with immunosuppression. Infections of C. albidus have been isolated in cats and marine mammals. Recent evidence suggests that the majority of infections produced by this pathogen are associated with biofilm growth, which is also related with increased resistance to antifungal agents. Therefore, there is a great need to search for alternative antifungal agents for these fungi. The search for new molecules is currently occurring from nanoparticle drugs of plant peptide origin. This article presents a brief review of the literature regarding the epidemiology of cryptococcosis, as well as fungal resistance and new alternatives for treatment.
Similar content being viewed by others
References
Lanjewar DN (2011) The spectrum of clinical and pathological manifestations of AIDS in a consecutive series of 236 autopsied cases in Mumbai, India. Pathol Res Int 2011:547618
Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP (2009) Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 104(3):513–521
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC (2010) Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50(3):291–322
Kantarcioğlu AS, Boekhout T, De Hoog GS, Theelen B, Yücel A, Ekmekci TR, Fries BC, Ikeda R, Koslu A, Altas K (2007) Subcutaneous cryptococcosis due to Cryptococcus diffluens in a patient with sporotrichoid lesions case report, features of the case isolate and in vitro antifungal susceptibilities. Med Mycol 45(2):173–181
Khawcharoenporn T, Apisarnthanarak A, Mundy LM (2007) Non-neoformans cryptococcal infections: a systematic review. Infection 35(2):51–58
Levitz SM, Specht CA (2006) The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res 6(4):513–524
Grechi J, Marinho-Carvalho M, Zancan P, Cinelli LP, Gomes AM, Rodrigues ML, Nimrichter L, Sola-Penna M (2011) Glucuronoxylomannan from Cryptococcus neoformans down-regulates the enzyme 6-phosphofructo-1-kinase of macrophages. J Biol Chem 286(17):14820–14829
Filiú WF, Wanke B, Agüena SM, Vilela VO, Macedo RC, Lazéra M (2002) Avian habitats as sources of Cryptococcus neoformans in the city of Campo Grande, Mato Grosso do Sul, Brazil. Rev Soc Bras Med Trop 35(6):591–595
Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, Marriott D, Pfeiffer T, Parr D, Byth K (2000) Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 31(2):499–508
Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Macdougall L, Boekhout T, Kwon-Chung KJ, Meyer W (2004) A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101(49):17258–17263
Byrnes EJ 3rd, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, Heitman J (2009) Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis 199(7):1081–1086
Centers for Disease Control and Prevention (CDC) (2010) Emergence of Cryptococcus gattii—Pacific Northwest, 2004–2010. MMWR Morb Mortal Wkly Rep 59(28):865–868
Goldman DL, Lee SC, Mednick AJ, Montella L, Casadevall A (2000) Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun 68(2):832–838
Jong A, Wu CH, Shackleford GM, Kwon-Chung KJ, Chang YC, Chen HM, Ouyang Y, Huang SH (2008) Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol 10(6):1313–1326
Jesus MS, Rodrigues WC, Barbosa G, Trilles L, Wanke B, Lazéra MdosS, Silva Md (2012) Cryptococcus neoformans carried by Odontomachus bauri ants. Mem Inst Oswaldo Cruz 107(4):466–469
Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9(2):189–195
Firacative C, Trilles L, Meyer W (2012) MALDI-TOF MS enables the rapid identification of the major molecular types within the Cryptococcus neoformans/C. gattii species complex. PLoS One 7(5):e37566
Nishikawa MM, Lazera MS, Barbosa GG, Trilles L, Balassiano BR, Macedo RC, Bezerra CC, Pérez MA, Cardarelli P, Wanke B (2003) Serotyping of 467 Cryptococcus neoformans isolates from clinical and environmental sources in Brazil: analysis of host and regional patterns. J Clin Microbiol 41(1):73–77
Kwon-Chung KJ (1991) The discovery of creatinine assimilation in Cryptococcus neoformans, and subsequent work on the characterization of the two varieties of C. neoformans. Zentralbl Bakteriol 275(3):390–393
Passoni LF, Wanke B, Nishikawa MM, Lazéra MS (1998) Cryptococcus neoformans isolated from human dwellings in Rio de Janeiro, Brazil: an analysis of the domestic environment of AIDS patients with and without cryptococcosis. Med Mycol 36(5):305–311
González-Hein G, González-Hein J, Díaz Jarabrán MC (2010) Isolation of Cryptococcus neoformans in dry droppings of captive birds in Santiago, Chile. J Avian Med Surg 24(3):227–236
Dixit A, Carroll SF, Qureshi ST (2009) Cryptococcus gattii: an emerging cause of fungal disease in North America. Interdiscip Perspect Infect Dis 2009:840452
Faria RO, Nascente PdaS, Meinerz AR, Cleff MB, Antunes TdeA, Silveira EdaS, Nobre MdeO, Meireles MC, Mello JR (2010) Occurrence of Cryptococcus neoformans in pigeon excrement in the city of Pelotas, State of Rio Grande do Sul. Rev Soc Bras Med Trop 43(2):198–200
Day JN, Hoang TN, Duong AV, Hong CT, Diep PT, Campbell JI, Sieu TP, Hien TT, Bui T, Boni MF, Lalloo DG, Carter D, Baker S, Farrar JJ (2011) Most cases of cryptococcal meningitis in HIV-uninfected patients in Vietnam are due to a distinct amplified fragment length polymorphism-defined cluster of Cryptococcus neoformans var. grubii VN1. J Clin Microbiol 49(2):658–664
Calvo BM, Colombo AL, Fischman O, Santiago A, Thompson L, Lazera M, Telles F, Fukushima K, Nishimura K, Tanaka R, Myiajy M, Moretti-Branchini ML (2001) Antifungal susceptibilities, varieties, and electrophoretic karyotypes of clinical isolates of Cryptococcus neoformans from Brazil, Chile, and Venezuela. J Clin Microbiol 39(6):2348–2350
Escandón P, Sánchez A, Martínez M, Meyer W, Castañeda E (2006) Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res 6(4):625–635
Severo LC, de Mattos Oliveira F, Londero AT (1999) Cryptococcosis due to Cryptococcus neoformans var. gattii in Brazilian patients with AIDS. Report of three cases. Rev Iberoam Micol 16(3):152–154
Byrnes EJ 3rd, Li W, Ren P, Lewit Y, Voelz K, Fraser JA, Dietrich FS, May RC, Chaturvedi S, Chaturvedi V, Heitman J (2011) A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog 7(9):e1002205
MacDougall L, Fyfe M, Romney M, Starr M, Galanis E (2011) Risk factors for Cryptococcus gattii infection, British Columbia, Canada. Emerg Infect Dis 17(2):193–199
Hagen F, Colom MF, Swinne D, Tintelnot K, Iatta R, Montagna MT, Torres-Rodriguez JM, Cogliati M, Velegraki A, Burggraaf A, Kamermans A, Sweere JM, Meis JF, Klaassen CH, Boekhout T (2012) Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis 18(10):1618–1624
Hagen F, Illnait-Zaragozi MT, Bartlett KH, Swinne D, Geertsen E, Klaassen CH, Boekhout T, Meis JF (2010) In vitro antifungal susceptibilities and amplified fragment length polymorphism genotyping of a worldwide collection of 350 clinical, veterinary, and environmental Cryptococcus gattii isolates. Antimicrob Agents Chemother 54(12):5139–5145
Georgi A, Schneemann M, Tintelnot K, Calligaris-Maibach RC, Meyer S, Weber R, Bosshard PP (2009) Cryptococcus gattii meningoencephalitis in an immunocompetent person 13 months after exposure. Infection 37(4):370–373
Litvintseva AP, Thakur R, Reller LB, Mitchell TG (2005) Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in Sub-Saharan Africa. J Infect Dis 192(5):888–892
Randhawa HS, Kowshik T, Chowdhary A, Preeti Sinha K, Khan ZU, Sun S, Xu J (2008) The expanding host tree species spectrum of Cryptococcus gattii and Cryptococcus neoformans and their isolations from surrounding soil in India. Med Mycol 46(8):823–833
Firacative C, Torres G, Rodríguez MC, Escandón P (2011) First environmental isolation of Cryptococcus gattii serotype B, from Cúcuta, Colombia. Biomedica 31(1):118–123
Martins LM, Wanke B, Lazéra MdosS, Trilles L, Barbosa GG, de Macedo RC, Cavalcanti MdoA, Eulálio KD, de Castro JA, da Silva AS, do Nacimento FF, Gouveia VA, do Monte SJ (2011) Genotypes of Cryptococcus neoformans and Cryptococcus gattii as agents of endemic cryptococcosis in Teresin, Piauí (northeastern Brazil). Mem Inst Oswaldo Cruz 106(6):725–730
Colom MF, Frasés S, Ferrer C, Jover A, Andreu M, Reus S, Sánchez M, Torres-Rodríguez JM (2005) First case of human cryptococcosis due to Cryptococcus neoformans var. gattii in Spain. J Clin Microbiol 43(7):3548–3550
McCurdy LH, Morrow JD (2003) Infections due to non-neoformans cryptococcal species. Compr Ther 29(2–3):95–101
Cheng MF, Chiou CC, Liu YC, Wang HZ, Hsieh KS (2001) Cryptococcus laurentii fungemia in a premature neonate. J Clin Microbiol 39(4):1608–1611
Averbuch D, Boekhoutt T, Falk R, Engelhard D, Shapiro M, Block C, Polacheck I (2002) Fungemia in a cancer patient caused by fluconazole-resistant Cryptococcus laurentii. Med Mycol 40(5):479–484
Shankar EM, Kumarasamy N, Bella D, Renuka S, Kownhar H, Suniti S, Rajan R, Rao UA (2006) Pneumonia and pleural effusion due to Cryptococcus laurentii in a clinically proven case of AIDS. Can Respir J 13(5):275–278
Furman-Kuklińska K, Naumnik B, Myśliwiec M (2009) Fungaemia due to Cryptococcus laurentii as a complication of immunosuppressive therapy—a case report. Adv Med Sci 54(1):116–119
Sugita T, Takashima M, Ikeda R, Nakase T, Shinoda T (2001) Intraspecies diversity of Cryptococcus albidus isolated from humans as revealed by sequences of the internal transcribed spacer regions. Microbiol Immunol 45(4):291–297
Kano R, Kitagawat M, Oota S, Oosumi T, Murakami Y, Tokuriki M, Hasegawa A (2008) First case of feline systemic Cryptococcus albidus infection. Med Mycol 46(1):75–77
Mcleland S, Duncan C, Spraker T, Wheeler E, Lockhart SR, Gulland F (2012) Cryptococcus albidus infection in a California sea lion (Zalophus californianus). J Wildl Dis 48(4):1030–1034
Leite DP Jr, Amadio JV, Martins ER, Simões SA, Yamamoto AC, Leal-Santos FA, Takahara DT, Hahn RC (2012) Cryptococcus spp isolated from dust microhabitat in Brazilian libraries. J Occup Med Toxicol 7(1):11
Sanglard D, Coste A, Ferrari S (2009) Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res 9(7):1029–1050
Jarvis JN, Dromer F, Harrison TS, Lortholary O (2008) Managing cryptococcosis in the immunocompromised host. Curr Opin Infect Dis 21(6):596–603
Sloan D, Dlamini S, Paul N, Dedicoat M (2008) Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev (4):CD005647
Catalán M, Montejo JC (2006) Systemic antifungals. Pharmacodynamics and pharmacokinetics. Rev Iberoam Micol 23(1):39–49
Polak A, Scholer HJ (1975) Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy 21(3–4):113–130
Trösken ER, Fischer K, Völkel W, Lutz WK (2006) Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 219(1–3):33–40
Kontoyiannis DP, Lewis RE (2002) Antifungal drug resistance of pathogenic fungi. Lancet 359(9312):1135–1144
Shao LC, Sheng CQ, Zhang WN (2007) Recent advances in the study of antifungal lead compounds with new chemical scaffolds. Yao Xue Xue Bao 42(11):1129–1136
Johnson E, Espinel-Ingroff A, Szekely A, Hockey H, Troke P (2008) Activity of voriconazole, itraconazole, fluconazole and amphotericin B in vitro against 1763 yeasts from 472 patients in the voriconazole phase III clinical studies. Int J Antimicrob Agents 32(6):511–514
Guerrero A, Fries BC (2008) Phenotypic switching in Cryptococcus neoformans contributes to virulence by changing the immunological host response. Infect Immun 76(9):4322–4331
Silva PR, Rabelo RA, Terra AP, Teixeira DN (2008) Susceptibility to antifungal agents among Cryptococcus neoformans varieties isolated from patients at a university hospital. Rev Soc Bras Med Trop 41(2):158–162
Andrade-Silva L, Ferreira-Paim K, Mora DJ, Silva PR, Andrade AA, Araujo NE, Pedrosa AL, Silva-Vergara ML (2013) Susceptibility profile of clinical and environmental isolates of Cryptococcus neoformans and Cryptococcus gattii in Uberaba, Minas Gerais, Brazil. Med Mycol [Epub ahead of print]
Andriole VT (1998) Current and future therapy of invasive fungal infections. Curr Clin Top Infect Dis 18:19–36
Illnait-Zaragozi MT, Martínez GF, Curfs-Breuker I, Fernández CM, Boekhout T, Meis JF (2008) In vitro activity of the new azole isavuconazole (BAL4815) compared with six other antifungal agents against 162 Cryptococcus neoformans isolates from Cuba. Antimicrob Agents Chemother 52(4):1580–1582
Abruzzo GK, Flattery AM, Gill CJ, Kong L, Smith JG, Pikounis VB, Balkovec JM, Bouffard AF, Dropinski JF, Rosen H, Kropp H, Bartizal K (1997) Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother 41(11):2333–2338
Espinel-Ingroff A (2009) Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009). Rev Iberoam Micol 26(1):15–22
White NJ (1998) Preventing antimalarial drug resistance through combinations. Drug Resist Updat 1(1):3–9
Rodríguez Tudela JL (1997) The resistance of opportunistic fungi to antifungals. Rev Clin Esp 197(Suppl 1):67–74
Pemán J, Cantón E, Espinel-Ingroff A (2009) Antifungal drug resistance mechanisms. Expert Rev Anti Infect Ther 7(4):453–460
Nosanchuk JD, Casadevall A (2003) The contribution of melanin to microbial pathogenesis. Cell Microbiol 5(4):203–223
Bryan RA, Jiang Z, Howell RC, Morgenstern A, Bruchertseifer F, Casadevall A, Dadachova E (2010) Radioimmunotherapy is more effective than antifungal treatment in experimental cryptococcal infection. J Infect Dis 202(4):633–637
Marichal P, Vanden Bossche H (1995) Mechanisms of resistance to azole antifungals. Acta Biochim Pol 42(4):509–516
Sanglard D, Ischer F, Monod M, Bille J (1997) Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143(Pt 2):405–416
Ferreira MJ, Gyémánt N, Madureira AM, Tanaka M, Koós K, Didziapetris R, Molnár J (2005) The effects of jatrophane derivatives on the reversion of MDR1- and MRP-mediated multidrug resistance in the MDA-MB-231 (HTB-26) cell line. Anticancer Res 25(6B):4173–4178
Espinel-Ingroff A (2008) Mechanisms of resistance to antifungal agents: yeasts and filamentous fungi. Rev Iberoam Micol 25(2):101–106
Monk BC, Niimi K, Lin S, Knight A, Kardos TB, Cannon RD, Parshot R, King A, Lun D, Harding DR (2005) Surface-active fungicidal D-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob Agents Chemother 49(1):57–70
Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, Tanabe K, Niimi M, Goffeau A, Monk BC (2009) Efflux-mediated antifungal drug resistance. Clin Microbiol Rev 22(2):291–321, Table of Contents
Prasad R, Gaur NA, Gaur M, Komath SS (2006) Efflux pumps in drug resistance of Candida. Infect Disord Drug Targets 6(2):69–83
Rodero L, Mellado E, Rodriguez AC, Salve A, Guelfand L, Cahn P, Cuenca-Estrella M, Davel G, Rodriguez-Tudela JL (2003) G484S amino acid substitution in lanosterol 14-alpha demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob Agents Chemother 47(11):3653–3656
Almeida AM, Matsumoto MT, Baeza LC, de Oliveira E Silva RB, Kleiner AA, Melhem MdeS, Mendes Giannini MJ; Laboratory Group on Cryptococcosis (2007) Molecular typing and antifungal susceptibility of clinical sequential isolates of Cryptococcus neoformans from Sao Paulo State, Brazil. FEMS Yeast Res 7(1):152–164
Sheng C, Miao Z, Ji H, Yao J, Wang W, Che X, Dong G, Lü J, Guo W, Zhang W (2009) Three-dimensional model of lanosterol 14 alpha-demethylase from Cryptococcus neoformans: active-site characterization and insights into azole binding. Antimicrob Agents Chemother 53(8):3487–3495
Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J (1995) Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 39(11):2378–2386
Marichal P, Vanden Bossche H, Odds FC, Nobels G, Warnock DW, Timmerman V, Van Broeckhoven C, Fay S, Mose-Larsen P (1997) Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother 41(10):2229–2237
Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martínez M, Calabrese D, Sanglard D, Patterson TF (2001) Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45(10):2676–2684
Vandeputte P, Larcher G, Bergès T, Renier G, Chabasse D, Bouchara JP (2005) Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob Agents Chemother 49(11):4608–4615
Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL (2003) A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 47(3):1120–1124
Mann PA, Parmegiani RM, Wei SQ, Mendrick CA, Li X, Loebenberg D, DiDomenico B, Hare RS, Walker SS, McNicholas PM (2003) Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14alpha-demethylase. Antimicrob Agents Chemother 47(2):577–581
Garcia-Effron G, Dilger A, Alcazar-Fuoli L, Park S, Mellado E, Perlin DS (2008) Rapid detection of triazole antifungal resistance in Aspergillus fumigatus. J Clin Microbiol 46(4):1200–1206
Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ (2009) Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother 53(7):2804–2815
Varma A, Kwon-Chung KJ (2010) Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 54(6):2303–2311
Sionov E, Lee H, Chang YC, Kwon-Chung KJ (2010) Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6(4):e1000848
Mondon P, Petter R, Amalfitano G, Luzzati R, Concia E, Polacheck I, Kwon-Chung KJ (1999) Heteroresistance to fluconazole and voriconazole in Cryptococcus neoformans. Antimicrob Agents Chemother 43(8):1856–1861
Bovers M, Hagen F, Kuramae EE, Hoogveld HL, Dromer F, St-Germain G, Boekhout T (2008) AIDS patient death caused by novel Cryptococcus neoformans × C. gattii hybrid. Emerg Infect Dis 14(7):1105–1108
Byrnes EJ 3rd, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 6(4):e1000850
Espinel-Ingroff A, Aller AI, Canton E, Castañón-Olivares LR, Chowdhary A, Cordoba S, Cuenca-Estrella M, Fothergill A, Fuller J, Govender N, Hagen F, Illnait-Zaragozi MT, Johnson E, Kidd S, Lass-Flörl C, Lockhart SR, Martins MA, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez T, Pfaller MA, Schell WA, St-Germain G, Trilles L, Turnidge J (2012) Cryptococcus neoformans–Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 56(11):5898–5906
Chong HS, Dagg R, Malik R, Chen S, Carter D (2010) In vitro susceptibility of the yeast pathogen Cryptococcus to fluconazole and other azoles varies with molecular genotype. J Clin Microbiol 48(11):4115–4120
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Bijie H, Dzierzanowska D, Klimko NN, Letscher-Bru V, Lisalova M, Muehlethaler K, Rennison C, Zaidi M; Global Antifungal Surveillance Group (2009) Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 47(1):117–123
Rex JH, Rinaldi MG, Pfaller MA (1995) Resistance of Candida species to fluconazole. Antimicrob Agents Chemother 39(1):1–8
Morschhäuser J (2010) Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 47(2):94–106
Martinez LR, Fries BC (2010) Fungal biofilms: relevance in the setting of human disease. Curr Fungal Infect Rep 4(4):266–275
Bach A (1996) Central venous catheter infections. Intensive Care Med 22(6):613
Martinez LR, Casadevall A (2006) Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun 74(11):6118–6123
Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL (2002) Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 49(6):973–980
Mukherjee PK, Zhou G, Munyon R, Ghannoum MA (2005) Candida biofilm: a well-designed protected environment. Med Mycol 43(3):191–208
Martinez LR, Casadevall A (2005) Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun 73(10):6350–6362
Mah TF, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9(1):34–39
de Aguiar Cordeiro R, Mourão CI, Rocha MF, de Farias Marques FJ, Teixeira CE, de Oliveira Miranda DF, Neto LV, Brilhante RS, de Jesus Pinheiro Gomes Bandeira T, Sidrim JJ (2013) Antifolates inhibit Cryptococcus biofilms and enhance susceptibility of planktonic cells to amphotericin B. Eur J Clin Microbiol Infect Dis 32(4):557–564
Robertson EJ, Wolf JM, Casadevall A (2012) EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl Environ Microbiol 78(22):7977–7984
Martinez LR, Mihu MR, Han G, Frases S, Cordero RJ, Casadevall A, Friedman AJ, Friedman JM, Nosanchuk JD (2010) The use of chitosan to damage Cryptococcus neoformans biofilms. Biomaterials 31(4):669–679
Pettit RK, Repp KK, Hazen KC (2010) Temperature affects the susceptibility of Cryptococcus neoformans biofilms to antifungal agents. Med Mycol 48(2):421–426
Quindós G, Del Palacio A, Pontón J (2007) Present and future of voriconazole in the treatment of invasive mycoses: the inseparable binomial diagnosis-treatment. Rev Iberoam Micol 24(3):179–180
Quindós G, Villar-Vidal M, Eraso E (2009) Activity of micafungin against Candida biofilms. Rev Iberoam Micol 26(1):49–55
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000) Atividade de extratos vegetais e fitofármacos sobre bactérias resistentes a antibióticos. Braz J Microbiol 31(4):247–256
Basso LA, da Silva LH, Fett-Neto AG, de Azevedo WF Jr, Moreira IdeS, Palma MS, Calixto JB, Astolfi Filho S, dos Santos RR, Soares MB, Santos DS (2005) The use of biodiversity as source of new chemical entities against defined molecular targets for treatment of malaria, tuberculosis, and T-cell mediated diseases—a review. Mem Inst Oswaldo Cruz 100(6):475–506
Rojas JJ, Ochoa VJ, Ocampo SA, Muñoz JF (2006) Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med 6:2
Cruz MC, Santos PO, Barbosa AM Jr, de Mélo DL, Alviano CS, Antoniolli AR, Alviano DS, Trindade RC (2007) Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J Ethnopharmacol 111(2):409–412
Zhu F, Ma XH, Qin C, Tao L, Liu X, Shi Z, Zhang CL, Tan CY, Chen YZ, Jiang YY (2012) Drug discovery prospect from untapped species: indications from approved natural product drugs. PLoS One 7(7):e39782
Gullo FP, Sardi JC, Santos VA, Sangalli-Leite F, Pitangui NS, Rossi SA, de Paula E Silva AC, Soares LA, Silva JF, Oliveira HC, Furlan M, Silva DH, Bolzani VS, Mendes-Giannini MJ, Fusco-Almeida AM (2012) Antifungal activity of maytenin and pristimerin. Evid Based Complement Alternat Med 2012:340787
de Carvalho Tavares L, Johann S, Maria de Almeida Alves T, Guerra JC, Maria de Souza-Fagundes E, Cisalpino PS, Bortoluzzi AJ, Caramori GF, de Mattos Piccoli R, Braibante HTS, Braibante MEF, Pizzolatti MG (2011) Quinolinyl and quinolinyl N-oxide chalcones: synthesis, antifungal and cytotoxic activities. Eur J Med Chem 46(9):4448–4456
Nondo RS, Mbwambo ZH, Kidukuli AW, Innocent EM, Mihale MJ, Erasto P, Moshi MJ (2011) Larvicidal, antimicrobial and brine shrimp activities of extracts from Cissampelos mucronata and Tephrosia villosa from coast region, Tanzania. BMC Complement Altern Med 11:33
Rangkadilok N, Tongchusak S, Boonhok R, Chaiyaroj SC, Junyaprasert VB, Buajeeb W, Akanimanee J, Raksasuk T, Suddhasthira T, Satayavivad J (2012) In vitro antifungal activities of longan (Dimocarpus longan Lour.) seed extract. Fitoterapia 83(3):545–553
Tocci N, Simonetti G, D’Auria FD, Panella S, Palamara AT, Valletta A, Pasqua G (2011) Root cultures of Hypericum perforatum subsp. angustifolium elicited with chitosan and production of xanthone-rich extracts with antifungal activity. Appl Microbiol Biotechnol 91(4):977–987
Clancy CJ, Nguyen MH (1998) The combination of amphotericin B and azithromycin as a potential new therapeutic approach to fusariosis. J Antimicrob Chemother 41(1):127–130
Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR (2000) Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 79(4):250–260
Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52(1):1
Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH (2004) Combination antifungal therapy. Antimicrob Agents Chemother 48(3):693–715
Serena C, Fernández-Torres B, Pastor FJ, Trilles L, Lazéra MdosS, Nolard N, Guarro J (2005) In vitro interactions of micafungin with other antifungal drugs against clinical isolates of four species of Cryptococcus. Antimicrob Agents Chemother 49(7):2994–2996
Han Y, Lee JH (2005) Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol Pharm Bull 28(3):541–544
Han Y (2007) Synergic effect of grape seed extract with amphotericin B against disseminated candidiasis due to Candida albicans. Phytomedicine 14(11):733–738
Saag MS, Graybill RJ, Larsen RA, Pappas PG, Perfect JR, Powderly WG, Sobel JD, Dismukes WE (2000) Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 30(4):710–718
Schwarz P, Dromer F, Lortholary O, Dannaoui E (2003) In vitro interaction of flucytosine with conventional and new antifungals against Cryptococcus neoformans clinical isolates. Antimicrob Agents Chemother 47(10):3361–3364
Leite FS (2010) Perfil fenotípico e de expressão de proteínas de Cryptococcus neoformans após tratamento com substâncias obtidas da planta Pterogyne nitens. Análises Clínicas, Faculdade de Ciências Farmacêuticas, UNESP, Araraquara
Suzano FR (2012) Estudo da atividade antifúngica de extratos e frações purificadas das plantas Serjania erecta radlk e Eclipta alba em linhagens de Cryptococcus neoformans. Biotecnologia, Universidade de Ribeirão Preto, Ribeirão Preto
Ahmad A, Khan A, Manzoor N (2012) Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur J Pharm Sci 48(1–2):80–86
Cabras T, Longhi R, Secundo F, Nocca G, Conti S, Polonelli L, Fanali C, Inzitari R, Petruzzelli R, Messana I, Castagnola M, Vitali A (2008) Structural and functional characterization of the porcine proline-rich antifungal peptide SP-B isolated from salivary gland granules. J Pept Sci 14(3):251–260
Gallis HA, Drew RH, Pickard WW (1990) Amphotericin B: 30 years of clinical experience. Rev Infect Dis 12(2):308–329
Clemons KV, Stevens DA (1998) Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother 42(4):899–902
Xu SF, Wang P, Liang ZX, Sun JP, Zhao XW, Li AM, Chen LA (2011) Investigation of inflammatory responses of pulmonary microvascular endothelial cells induced by lipopolysaccharide and mechanism. Zhonghua Jie He He Hu Xi Za Zhi 34(11):816–820
Wang H, Xu K, Liu L, Tan JP, Chen Y, Li Y, Fan W, Wei Z, Sheng J, Yang YY, Li L (2010) The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials 31(10):2874–2881
Yaguchi T, Takizawa K, Taguchi H, Tanaka R, Kubota T, Kubota Y, Kubota M, Fukushima K (2007) Antifungal activity of the novel adduct, GX-95, of silver with nanometer-scale particles to peptidic hydrolysates from collagen. Nihon Ishinkin Gakkai Zasshi 48(2):97–100
Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E (2007) Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob Agents Chemother 51(8):2929–2936
Soares BM, Alves OA, Ferreira MV, Amorim JC, Sousa GR, Silveira LdeB, Prates RA, Avila TV, Baltazar LdeM, de Souza DdaG, Santos DA, Modolo LV, Cisalpino PS, Pinotti M (2011) Cryptococcus gattii: in vitro susceptibility to photodynamic inactivation. Photochem Photobiol 87(2):357–364
Rodrigues GB, Primo FL, Tedesco AC, Braga GU (2012) In vitro photodynamic inactivation of Cryptococcus neoformans melanized cells with chloroaluminum phthalocyanine nanoemulsion. Photochem Photobiol 88(2):440–447
Antachopoulos C, Walsh TJ (2012) Immunotherapy of Cryptococcus infections. Clin Microbiol Infect 18(2):126–133
Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL Jr (2012) Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J Immunol 189(8):4060–4068
Kawakami K, Tohyama M, Xie Q, Saito A (1996) IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol 104(2):208–214
Clemons KV, Lutz JE, Stevens DA (2001) Efficacy of recombinant gamma interferon for treatment of systemic cryptococcosis in SCID mice. Antimicrob Agents Chemother 45(3):686–689
Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G (1998) Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 66(10):4994–5000
Clemons KV, Brummer E, Stevens DA (1994) Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother 38(3):460–464
Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker LG, Wood R, Harrison TS (2012) Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26(9):1105–1113
Qureshi MH, Zhang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto M, Kawakami K (1999) Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol 29(2):643–649
Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A (1997) Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun 65(9):3594–3599
Khan AA, Jabeen M, Chauhan A, Owais M (2012) Vaccine potential of cytosolic proteins loaded fibrin microspheres of Cryptococcus neoformans in BALB/c mice. J Drug Target 20(5):453–466
Casadevall A (2006) The third age of antimicrobial therapy. Clin Infect Dis 42(10):1414–1416
Pachl J, Svoboda P, Jacobs F, Vandewoude K, van der Hoven B, Spronk P, Masterson G, Malbrain M, Aoun M, Garbino J, Takala J, Drgona L, Burnie J, Matthews R; Mycograb Invasive Candidiasis Study Group (2006) A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42(10):1404–1413
Taborda CP, Casadevall A (2001) Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J Immunol 166(3):2100–2107
Mukherjee J, Kozel TR, Casadevall A (1998) Monoclonal antibodies reveal additional epitopes of serotype D Cryptococcus neoformans capsular glucuronoxylomannan that elicit protective antibodies. J Immunol 161(7):3557–3568
Rosas AL, Nosanchuk JD, Casadevall A (2001) Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect Immun 69(5):3410–3412
Mukherjee J, Scharff MD, Casadevall A (1992) Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun 60(11):4534–4541
Mukherjee J, Zuckier LS, Scharff MD, Casadevall A (1994) Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother 38(3):580–587
Mukherjee J, Feldmesser M, Scharff MD, Casadevall A (1995) Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob Agents Chemother 39(7):1398–1405
Larsen RA, Pappas PG, Perfect J, Aberg JA, Casadevall A, Cloud GA, James R, Filler S, Dismukes WE (2005) Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother 49(3):952–958
Jiang Z, Bryan RA, Morgenstern A, Bruchertseifer F, Casadevall A, Dadachova E (2012) Treatment of early and established Cryptococcus neoformans infection with radiolabeled antibodies in immunocompetent mice. Antimicrob Agents Chemother 56(1):552–554
Marongiu B, Piras A, Porcedda S, Falconieri D, Maxia A, Frau MA, Gonçalves MJ, Cavaleiro C, Salgueiro L (2012) Isolation of the volatile fraction from Apium graveolens L. (Apiaceae) by supercritical carbon dioxide extraction and hydrodistillation: chemical composition and antifungal activity. Nat Prod Res [Epub ahead of print]
Fukuyama N, Shibuya M, Orihara Y (2012) Antimicrobial polyacetylenes from Panax ginseng hairy root culture. Chem Pharm Bull (Tokyo) 60(3):377–380
Lago JH, Souza ED, Mariane B, Pascon R, Vallim MA, Martins RC, Baroli AA, Carvalho BA, Soares MG, dos Santos RT, Sartorelli P (2011) Chemical and biological evaluation of essential oils from two species of Myrtaceae—Eugenia uniflora L. and Plinia trunciflora (O. Berg) Kausel. Molecules 16(12):9827–9837
Patel KD, Scarano FJ, Kondo M, Hurta RA, Neto CC (2011) Proanthocyanidin-rich extracts from cranberry fruit (Vaccinium macrocarpon Ait.) selectively inhibit the growth of human pathogenic fungi Candida spp. and Cryptococcus neoformans. J Agric Food Chem 59(24):12864–12873
Fukuyama N, Ino C, Suzuki Y, Kobayashi N, Hamamoto H, Sekimizu K, Orihara Y (2011) Antimicrobial sesquiterpenoids from Laurus nobilis L. Nat Prod Res 25(14):1295–1303
Funari CS, Gullo FP, Napolitano A, Carneiro RL, Mendes-Giannini MJ, Fusco-Almeida AM, Piacente S, Pizza C, Silva DH (2012) Chemical and antifungal investigations of six Lippia species (Verbenaceae) from Brazil. Food Chem 135(3):2086–2094
Regasini LO, Pivatto M, Scorzoni L, Benaducci T, Fusco-Almeida AM, Mendes-Giannini MJS, Barreiro EJ, Silva DHS, da Silva Bolzani V (2010) Antimicrobial activity of Pterogyne nitens Tul., Fabaceae, against opportunistic fungi. Rev Bras Farmacogn 20(5):706–711
Conflict of interest
The authors declare that they have no conflict of interest.
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gullo, F.P., Rossi, S.A., Sardi, J.C.O. et al. Cryptococcosis: epidemiology, fungal resistance, and new alternatives for treatment. Eur J Clin Microbiol Infect Dis 32, 1377–1391 (2013). https://doi.org/10.1007/s10096-013-1915-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1915-8