Abstract
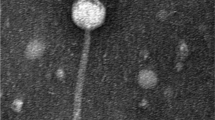
The main goal of this study was to evaluate the efficiency of phage therapy against one of the most common multidrug-resistant (MDR) agents of skin infections, Pseudomonas aeruginosa. A phage suspension [108 plaque-forming units (PFU) mL−1] was obtained using the clinical strain P. aeruginosa 709 as the host. The ability of the phage to inactivate P. aeruginosa was evaluated in vitro and ex vivo (human skin), using a multiplicity of infection (MOI) of 0.5 to 50. In the presence of the phage, the density of P. aeruginosa 709 [105 colony-forming units (CFU) mL−1] in the human skin decreased by 4 logs after 2 h of incubation. The application of a second dose of phage did not increase the efficiency of the therapy. This study indicates that the topical application of phage PA709 efficiently inactivates MDR P. aeruginosa 709. The high efficiency in the inactivation of MDR P. aeruginosa 709, its considerable host range (infection of 30 % of the P. aeruginosa isolates) and its high stability in buffer and ex vivo human skin make this phage very promising for the treatment of P. aeruginosa skin infections. The phage–bacteria interactions were examined in vitro and in ex vivo in order to provide a basis for the selection of the most suitable protocol for subsequent in vivo experiments.





Similar content being viewed by others
References
Wróblewska M (2006) Novel therapies of multidrug-resistant Pseudomonas aeruginosa and Acinetobacter spp. infections: the state of the art. Arch Immunol Ther Exp (Warsz) 54:113–120
Pai H, Kim JW, Kim J, Lee JH, Choe KW, Gotoh N (2001) Carbapenem resistance mechanisms in Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 45:480–484
Comeau AM, Hatfull GF, Krisch HM, Lindell D, Mann NH, Prangishvili D (2008) Exploring the prokaryotic virosphere. Res Microbiol 159:306–313
Sulakvelidze A, Alavidze Z, Morris JG Jr (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659
Housby JN, Mann NH (2009) Phage therapy. Drug Discov Today 14:536–540
Levin BR, Bull JJ (2004) Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2:166–173
Kumari S, Harjai K, Chhibber S (2010) Evidence to support the therapeutic potential of bacteriophage Kpn5 in burn wound infection caused by Klebsiella pneumoniae in BALB/c mice. J Microbiol Biotechnnol 20:935–941
Heo YJ, Lee YR, Jung HH, Lee JE, Ko GP, Cho YH (2009) Antibacterial efficacy of phages against Pseudomonas aeruginosa infections in mice and Drosophila melanogaster. Antimicrob Agents Chemother 53:2469–2474
Andreatti Filho RL, Higgins JP, Higgins SE, Gaona G, Wolfenden AD, Tellez G, Hargis BM (2007) Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar Enteritidis in vitro and in vivo. Poultry Sci 86:1904–1909
Wiggins BA, Alexander M (1985) Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol 49:19–23
Bull JJ, Levin BR, DeRouin T, Walker N, Bloch CA (2002) Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol 2:35
Goode D, Allen VM, Barrow PA (2003) Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol 69:5032–5036
Huff WE, Huff GR, Rath NC, Balog JM, Donoghue AM (2005) Alternatives to antibiotics: utilization of bacteriophage to treat colibacillosis and prevent foodborne pathogens. Poult Sci 84:655–659
Smith HW, Huggins MB (1982) Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol 128:307–318
Watanabe R, Matsumoto T, Sano G, Ishii Y, Tateda K, Sumiyama Y, Uchiyama J, Sakurai S, Matsuzaki S, Imai S, Yamaguchi K (2007) Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother 51:446–452
McVay CS, Velásquez M, Fralick JA (2007) Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother 51:1934–1938
Jikia D, Chkhaidze N, Imedashvili E, Mgaloblishvili I, Tsitlanadze G, Katsarava R, Glenn Morris J Jr, Sulakvelidze A (2005) The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin Exp Dermatol 30:23–26
Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clin Microbiol Rev 19:403–434
Rossolini GM, Mantengoli E, Docquier JD, Musmanno RA, Coratza G (2007) Epidemiology of infections caused by multiresistant gram-negatives: ESBLs, MBLs, panresistant strains. New Microbiol 30:332–339
Bergogne-Bérézin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 9:148–165
Wang J, Hu B, Xu M, Yan Q, Liu S, Zhu X, Sun Z, Reed E, Ding L, Gong J, Li QQ, Hu J (2006) Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J Mol Med 17:309–317
Soothill JS (1994) Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns 20:209–211
Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L, Lavigne R, Volckaert G, Mattheus W, Verween G, De Corte P, Rose T, Jennes S, Zizi M, De Vos D, Vaneechoutte M (2009) Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944
Rademaker JLW, Louws FJ, Versalovic J, de Bruijn FJ (2004) Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting. In: Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans AD, van Elsas JD (eds) Molecular microbial ecology manual. Volumes 1 and 2, 2nd edn. Kluwer Academic Publishers, Dordrecht
Adams MH (1959) Bacteriophages. Interscience Publishers, New York
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66:5488–5491
Ackermann HW (2007) 5500 Phages examined in the electron microscope. Arch Virol 152:227–243
Ackermann HW, Cartier C, Slopek S, Vieu JF (1988) Morphology of Pseudomonas aeruginosa typing phages of the Lindberg set. Ann Inst Pasteur Virol 139:389–404
Lavigne R, Darius P, Summer EJ, Seto D, Mahadevan P, Nilsson AS, Ackermann HW, Kropinski AM (2009) Classification of Myoviridae bacteriophages using protein sequence similarity. BMC Microbiol 9:224
Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L (2011) Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLoS One 6:e16963
Ahiwale S, Tamboli N, Thorat K, Kulkarni R, Ackermann H, Kapadnis B (2011) In vitro management of hospital Pseudomonas aeruginosa biofilm using indigenous T7-Like lytic phage. Curr Microbiol 62:335–340
Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM (2010) Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 54:397–404
Oliveira A, Sillankorva S, Quinta R, Henriques A, Sereno R, Azeredo J (2009) Isolation and characterization of bacteriophages for avian pathogenic E. coli strains. J Appl Microbiol 106:1919–1927
Sillankorva S, Oliveira R, Vieira MJ, Sutherland IW, Azeredo J (2004) Bacteriophage Phi S1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling 20:133–138
Skurnik M, Strauch E (2006) Phage therapy: facts and fiction. Int J Med Microbiol 296:5–14
Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR (2002) Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun 70:204–210
Acknowledgements
The authors want to thank the microbiology laboratories of the University Hospitals of Coimbra, the Local Health Unit of Matosinhos, the Hospital Infante Dom Pedro and the Avelab Laboratory in Aveiro for providing the bacterial isolates used in this study. The University of Aveiro and Centre for Environmental and Marine Studies (CESAM, Aveiro, Portugal) funded this investigation. Financial support to Y.J. Silva (SFRH/BD/65147/2009) was provided by the Portuguese Foundation for Science and Technology (FCT) in the form of a PhD grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vieira, A., Silva, Y.J., Cunha, Â. et al. Phage therapy to control multidrug-resistant Pseudomonas aeruginosa skin infections: in vitro and ex vivo experiments. Eur J Clin Microbiol Infect Dis 31, 3241–3249 (2012). https://doi.org/10.1007/s10096-012-1691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1691-x