Abstract
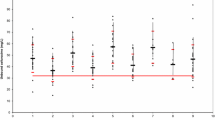
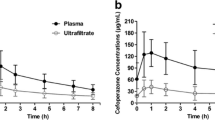
The purpose of this investigation was to study the effects of renal function on the pharmacokinetics and pharmacodynamics (PK-PD) of free cefazolin administered prophylactically in cardiothoracic surgery. Patients received an initial 2-g dose of cefazolin, followed by 1-g doses 6, 12, 18 and 24 h after the first dose. In patients who underwent cardiopulmonary bypass, 1 g was added to the priming solution. In 35 patients with a normal estimated creatinine clearance (CLcr) ≥50 ml/min, a free cefazolin concentration <4 μg/ml was observed in 11.4, 5.7 and 54.3% of patients before the second dose, at the end and 24 h after operation, respectively. In contrast, only 7.4% of 27 patients with CLcr <49 ml/min had a free cefazolin concentration <4 μg/ml 24 h after the operation. There was a high negative correlation between CLcr and time above the target minimal inhibitory concentration (MIC) when the CLcr was <50 ml/min (r 2 = 0.807), and no correlation when the CLcr was ≥50 ml/min. Renal function has a significant impact on the PK-PD of prophylactic cefazolin in cardiothoracic surgery. The postoperative drug dosing intervals should be <6 h in order to achieve a 100% time above the MIC in patients with CLcr ≥ 50 ml/min.


Similar content being viewed by others
References
Zelenitsky SA, Silverman RE, Duckworth H, Harding GK (2000) A prospective, randomized, double-blind study of single high dose versus multiple standard dose gentamicin both in combination with metronidazole for colorectal surgical prophylaxis. J Hosp Infect 46:135–40
Zelenitsky SA, Ariano RE, Harding GK, Silverman RE (2002) Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob Agents Chemother 46(9):3026–30
Harbarth S, Samore MH, Lichtenberg D, Carmeli Y (2000) Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 101:2916–2921
Prokuski L (2008) Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg 16:283–293
Gyssens IC (1999) Preventing postoperative infections: current treatment recommendations. Drugs 57:175–185
Nightingale CH, Greene DS, Quintiliani R (1975) Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci 64:1899–1926
Kirby WM, Regamey C (1973) Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis 128(Suppl):S341–346
Bergan T, Brodwall EK, Ørjavik O (1977) Pharmacokinetics of cefazolin patients with normal and impaired renal function. J Antimicrob Chemother 3:435–443
Craig WA (1998) Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10
Schaper KJ, Schubert S, Dalhoff A (2005) Kinetics and quantification of antibacterial effects of beta-lactams, macrolides, and quinolones against gram-positive and gram-negative RTI pathogens. Infection 33(Suppl 2):3–14
Nicolau DP, Quintiliani R (1994) Choosing between the new cephalosporin antibiotics: a pharmacodynamic approach. Pharmacoeconomics 5(Suppl 2):34–39
Bechtel TP, Slaughter RL, Moore TD (1980) Seizures associated with high cerebrospinal fluid concentrations of cefazolin. Am J Hosp Pharm 37:271–273
Bratzler DW, Houck PM (2004) Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 38:1706–1715
Edmiston CE, Krepel C, Kelly H, Larson J, Andris D, Hennen C, Nakeeb A, Wallace JR (2004) Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery 136:738–747
Swoboda SM, Merz C, Kostuik J, Trentler B, Lipsett PA (1996) Does intraoperative blood loss affect antibiotic serum and tissue concentrations? Arch Surg 131:1165–1171
Caffarelli AD, Holden JP, Baron EJ, Lemmens HJ, D’Souza H, Yau V, Olcott C 4th, Reitz BA, Miller DC, van der Starre PJ (2006) Plasma cefazolin levels during cardiovascular surgery: effects of cardiopulmonary bypass and profound hypothermic circulatory arrest. J Thorac Cardiovasc Surg 131:1338–1343
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Fukumoto A, Yamagishi M, Doi K, Ogawa M, Inoue T, Hashimoto S, Yaku H (2006) Hemodiafiltration during cardiac surgery in patients on chronic hemodialysis. J Card Surg 21:553–558
Nygard G, Wahba Khalil SK (1984) An isocratic HPLC method for the determination of cephalosporins in plasma. J Liquid Chromatogr 7:1461–1475
Bergamini TM, Polk HC Jr (1989) Pharmacodynamics of antibiotic penetration of tissue and surgical prophylaxis. Surg Gynecol Obstet 168:283–289
Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C; Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons (2007) The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg 83:1569–1576
Brown GR (1993) Cephalosporin–probenecid drug interactions. Clin Pharmacokinet 24:289–300
Hori R, Okumura K, Kamiya A, Nihira H, Nakano H (1983) Ampicillin and cephalexin in renal insufficiency. Clin Pharmacol Ther 34:792–798
Madhavan T, Yaremchuk K, Levin N, Fisher E, Cox F, Burch K, Haas E, Pohlod D, Quinn EL (1975) Effects of renal failure and dialysis on cefazolin pharmacokinetics. Antimicrob Agents Chemother 8:63–66
Lehot JJ (2004) Cefazolin prophylaxis during cardiac operations. Ann Thorac Surg 77:755–756
Drusano GL (2003) Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis 36(Suppl1):S42–S50
McKinnon PS, Paladino JA, Schentag JJ (2008) Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351
Acknowledgements
We thank Astellas Pharma Inc., Tokyo, Japan, and Shionogi & Co., Ltd., Osaka, Japan, for kindly supplying us with cefazolin and cephalexin, respectively. We also thank the staff of the Department of Pharmacokinetics at Kyoto Pharmaceutical University, School of Pharmacy, for their contributions to this study, and Yukimi Shibata, M.E., from the Kyoto Prefectural University of Medicine, University Hospital, for reviewing the manuscript.
Disclosure
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kosaka, T., Hosokawa, K., Shime, N. et al. Effects of renal function on the pharmacokinetics and pharmacodynamics of prophylactic cefazolin in cardiothoracic surgery. Eur J Clin Microbiol Infect Dis 31, 193–199 (2012). https://doi.org/10.1007/s10096-011-1293-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1293-z