Abstract
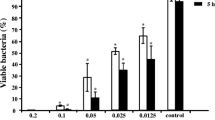
The present study focused on the antibacterial and biofilm inhibitory potential of 4-epi-pimaric acid isolated from aerial parts (stem and leaves) of Aralia cachemirica L. (Araliaceae) against oral cavity pathogens. 4-epi-Pimaric acid exhibited minimum inhibitory concentration (MIC) in the range of 4–16 μg/ml and minimum bactericidal concentration (MBC) two- to four-folds higher than MIC. There was significant inhibition in the biofilm formation by Streptococcus mutans on the saliva coated surface (P < 0.05), and confocal microscopy revealed that 4-epi-pimaric acid inhibited the clumping and attachment of S. mutans. At 8 × MIC concentration, it significantly prevented the pH drop and reduced S. mutans biofilms (P < 0.05). Increased propidium iodide staining and leakage of 260- and 280-nm absorbing material by 4-epi-pimaric acid treated cells of S. mutans suggested that it probably causes disruption of the cytoplasmic membrane structure. It also exhibited significant suppression of TNF-α expression in human neutrophils, suggestive of its anti-inflammatory activity. Furthermore, the compound was found to be significantly safe (IC50 >100 μg/ml) in the MTT assay on AML-12 cell lines. In conclusion, 4-epi-pimaric acid showed promising antibacterial, anti-biofilm and anti-inflammatory potency and this compound can be exploited for therapeutic application in oral microbial infections.









Similar content being viewed by others
References
Islam B, Khan SN, Khan AU (2007) Dental caries: from infection to prevention. Med Sci Monit 13:196–203
Nakano K, Nomura R, Nemoto H, Mukai T, Yoshioka H, Shudo Y, Hata H, Toda K, Taniguchi K, Amano A, Ooshima T (2007) Detection of novel serotype k Streptococcus mutans in infective endocarditis patients. J Med Microbiol 56:1413–1415
Li X, Kolltveit KM, Tronstad L, Olsen I (2000) Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558
Kolenbrander PE (2000) Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54:413–437
Hamada S, Slade HD (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44:331–384
Kuramitsu HK (1993) Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med 4:159–176
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380
Gibbons RJ, Qureshi JV (1976) Microbial aspects of dental caries. In: Washington, DC: information retrieval. Al-Hashimi, I. Levine, M.J (1989) Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol 34:289–295
Mogi M, Hiraoka BY, Harada M (1986) Analysis and identification of human parotid salivary proteins by micro two-dimensional electrophoresis and Western blot techniques. Arch Oral Biol 31:337–339
Busscher HJ, Cowan MM, Van der Mei HC (1992) On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol Rev 8:199–209
Wang X, Yao X, Zhu Z, Tang T, Dai K, Sadovskaya I, Flahaut S, Jabbouri S (2009) Effect of berberine on Staphylococcus epidermidis biofilm formation. Int J Antimicrob Agents 34:60–66
Koo H, Jeon JG (2009) Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilms. Adv Dent Res 21:63–68
Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG (2000) The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tree oil). J Appl Microbiol 88:170–175
Morgan TD, Beezer AE, Mitchell JC, Bunch AW (2001) A microcalorimetric comparison of the anti-Streptococcus mutans efficacy of plant extracts and antimicrobial agent in oral hygiene formulations. J Appl Microbiol 90:53–58
Sharma S, Khan IA, Ali I, Ali F, Kumar M, Kumar A, Johri RK, Abdullah ST, Bani S, Pandey A, Suri KA, Gupta BD, Satti NK, Dutt P, Qazi GN (2009) Evaluation of the antimicrobial, antioxidant, and anti-inflammatory activities of hydroxychavicol for its potential use as an oral care agent. Antimicrob Agents Chemother 53:216–222
Slots J, Rams TE (1990) Antibiotics in periodontal therapy: advantages and disadvantages. Oral Microbiol Immunol 17:479–493
Reddy MV, Thota N, Sangwan PL, Malhotra P, Ali F, Khan IA, Chimni SS, Koul S (2010) Novel bisstyryl derivatives of bakuchiol: targeting oral cavity pathogens. Eur J Med Chem 45:3125–3134
Frodin DG, Govaerts R (2003) World checklist and bibliography of Araliaceae. Kew, Royal Botanic Gardens, p 444
Wen J (2004) Systematics and biogeography of Aralia L. sect. Dimorphanthus (Miq.) Miq. (Araliaceae). Cathaya 15:1–187
Bown D (1995) Encyclopaedia of herbs and their uses. Dorling Kindersley, London
Weiner MA (1980) Earth medicine, earth Food. Ballantine, New York
Bhat ZA, Ansari SH, Mukhtar HM, Naved T, Khan NA, Chashoo IA (2005) Anti hyperglycemic activity of the alcoholic extract of Aralia cachemirica Decne roots. J Nat Rem 5:160–164
Verma RS, Padalia RC, Yadav A, Chauhan A (2010) Essential oil composition of Aralia cachemirica from Uttarakhand, India. Rec Nat Prod 4:163–166
Sangwan PL, Koul S, Khan IA, Raja AF, Qazi GN (2008) Antibacterial constituent of Aralia cachemiric. Proceedings of Asian Symposium on Medicinal Plants, Spices and other Natural Products (ASOMPS) XIII, Abstract 15, p 107, IICT. Hyderabad, India
Porto TS, Rangel R, Furtado NAJC, de Carvalho TC, Martins CHG, Veneziani RCS, Costa FBD, Vinholis AHC, Cunha WR, Heleno VCG, Ambrosio SR (2009) Pimarane-type diterpenes: antimicrobial activity against oral pathogens. Molecules 14:191–199
Clinical and Laboratory Standards Institute (CLSI) (2007) Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th edn. CLSI document M11-A7 (ISBN 1–56238–626–3). Clinical and Laboratory Standards Institute, Wayne, Pennsylvania USA
Eliopoulus GM, Moellering RCJ (1996) Antimicrobial combinations. In: Lorian V (ed) Antibiotics in laboratory medicine, 4th edn. The Williams & Wilkins Co., Baltimore, MD, pp 330–396
Craig WA, Gudmundsson S (1996) Postantibiotic effect. In: Lorian V (ed) Antibiotics in laboratory medicine, 4th edn. Williams and Wilkins Co, Baltimore, MD, pp 296–299
Shellis RP, Addy M, Rees GD (2005) In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. J Dent 33:313–324
Wei GX, Campagna AN, Bokek LA (2006) Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 57:1100–1109
Islam B, Khan SN, Haque I, Alam M, Mushfiq M, Khan AU (2008) Novel anti-adherence activity of mulberry leaves: inhibition of Streptococcus mutans biofilm by 1-deoxynojirimycin isolated from Morus alba. J Antimicrob Chemother 62:751–757
Dorsey WC, Tchounwou PB, Sutton D (2004) Mitogenic and cytotoxic effects of pentachlorophenol to AML 12 mouse hepatocytes. Int J Environ Res Public Health 1:100–105
Wenkert E, Afonso A, Beak P, Carney RWJ, Jeffs P, McChesney W (1965) The proton magnetic resonance spectral characteristics of tricyclic diterpenic substances. J Org Chem 30:713–722
Sy L-K, Brown GD (1998) Abietane Diterpenes from Illicium angustisepalum. J Nat Prod 61:907–912
Li YH, Hanna MN, Svensater G, Ellen PR, Cvitkovitch DG (2001) Cell density modulates acid adaptation in Streptococcus mutans: implication for survival in biofilm. J Bacteriol 183:6875–6884
Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, Hong EK (2005) Water extract of Aralia elata prevents cataractogenesis in-vitro and in-vivo. J Ethnopharmacol 101:49–54
Dang NH, Zhang X, Zheng M, Son KH, Chang HW, Kim HP, Bae K, Kang SS (2005) Inhibitory constituents against cyclooxygenases from Aralia cordata Thunb. Arch Pharm Res 28:28–33
Marsh PD (2000) Oral ecology and its impact on oral microbial diversity. In: Kuramitsu HK, Ellen RP (eds) Oral bacterial ecology: the molecular basis. Horizon Scientific Press, Bymondham, Norfolk, United Kingdom, pp 11–65
Nobbs AH, Lamont RJ, Jenkinson HF (2009) Streptococcus adherence and colonization. Microbiol Mol Biol Rev 73:407–450
Sedlacek MJ, Walker C (2007) Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol Immunol 22:333–339
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (Tea Tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46:1914–1920
Kang O-H, Chae H-S, Choi J-G, Oh Y-C, Lee Y-S, Kim J-H, Seung M-J, Jang H-J, Bae K-H, Lee J-H, Shin D-W, Kwon DY (2008) ent-pimara-8(14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-κB activation and mitogen-activated protein kinase pathways. Eur J Pharmacol 601:179–185
Acknowledgments
We gratefully acknowledge the Corps Dental Hospital, Jammu, India for providing clinical samples. This work was supported by a grant from Colgate-Palmolive Company, New Jersey, USA, under the grant no. SSP0415.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, F., Sangwan, P.L., Koul, S. et al. 4-epi-Pimaric acid: a phytomolecule as a potent antibacterial and anti-biofilm agent for oral cavity pathogens. Eur J Clin Microbiol Infect Dis 31, 149–159 (2012). https://doi.org/10.1007/s10096-011-1287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1287-x