Abstract
The life history and feeding biology of the bamboo powderpost beetle Dinoderus minutus remain poorly understood because the beetles’ oviposition, development, and feeding take place inside bamboo culms. In this study, X-ray computed tomography (CT) was employed to nondestructively quantify the progression of larval body size and tunnel size from the first instar to pupation. Eggs of D. minutus laid between laminates of nutrient-containing filter paper were easily collected. The newly hatched larvae were inoculated singly into pieces of internodes of madake (Phyllostachys bambusoides). The pieces were scanned using a microfocus X-ray CT system every 3–5 days to visualize the beetles’ bodies and tunnels with resolutions of 18–60 μm/voxel. CT scans were continued after adult eclosion to analyze pre-mating adult feeding. The collected eggs were 0.84 ± 0.06 mm (mean ± SD) in length and the egg duration lasted 5.0 ± 0.8 days. Based on CT images, the larvae grew to 3.53 ± 0.23 mm in body length and turned into pupae of 3.42 ± 0.09 mm. The larvae bored tunnels with a length of 80.2 ± 4.8 mm and a volume of 68.0 ± 7.0 mm3 over the larval period of 61 ± 11 days. Newly emerged adults remained in the bamboo pieces to feed before making exit holes in 8 ± 1 days after adult eclosion. During this period, they bored tunnels at rates of 2.64 ± 0.58 mm/day in length and 4.87 ± 1.10 mm3/day in volume.
Similar content being viewed by others
Introduction
Bamboo is an abundant natural resource in many regions, and bamboo culms can serve as a strong material with high machinability and esthetic values. In Japan, bamboo culms have been used as construction and decorative materials in many traditional wooden houses, as well as for furniture and craft products. However, bamboo culms are susceptible to biodeterioration, even in seasoned conditions, and can be easily attacked by insects, thus limiting their usage.
The bamboo powderpost beetle Dinoderus minutus (Fabricius) (Coleoptera: Bostrichidae) is one of the most significant pests of felled bamboo in Japan [1,2,3]. Attack begins by adults entering into the culms from cut surfaces and ovipositing. The hatched larvae bore tunnels to take in nutrients, such as starch, from parenchyma tissues and develop into pupae. Adults also feed on bamboo after eclosion and leave via exit holes to mate [1,2,3]. In the processes of larval and adult feeding and oviposition, bamboo tissues are turned into powder called frass, reducing the strength and esthetic qualities of the culms.
Infestation of D. minutus can begin by adult beetles entering into and ovipositing inside bamboo culms during the process of commercial timber manufacture. To prevent this, protection measures are necessary. When the presence of attack is suspected, disinfestation measures, such as heat treatment and fumigation, are also needed. Protection and disinfestation methods that do not rely heavily on chemicals are desired because of associated impacts on the environment and human health. Infestation can also occur when adult beetles enter constructed bamboo culms or manufactured products in use. Measures against this include early detection and assessment of attack and application of proper treatments, such as replacement of damaged parts or disinfestation. Establishment of these control measures requires detailed understanding of the biology of D. minutus, including information on life history and feeding behavior.
Dinoderus minutus spend most of their life cycle in bamboo culms where they feed, develop, and oviposit, making analysis difficult. Recent studies have sought to reveal the process of larval development [4,5,6] and reproductive capacity [7] of D. minutus. However, these studies were based on the direct dissection of bamboo culms [4, 6], which may have affected the natural growth or behavior of the beetles, or on tests using artificial diets [5, 7], in which the beetles’ growth or behavior may be different from those in bamboo culms.
We previously utilized X-ray computed tomography (CT) to nondestructively trace the growth and tunneling of D. minutus larvae inside infested bamboo pieces over time, and reported that the developmental stages (larva, prepupa, pupa, and adult) were clearly distinguishable in the CT images and their bamboo consumption was assessed quantitatively [8]. However, because we were unable to locate eggs or newly hatched larvae, the complete process of egg-to-adult development could not be determined. To do so, an easy method to collect eggs was desired.
In this study, first, a method for egg collecting was clarified. Then, larvae immediately after hatching were inoculated in bamboo pieces, and X-ray CT was used to observe and quantify the development and feeding of D. minutus from hatching to pupation and adult eclosion. The feeding of newly emerged pre-mating adults was also analyzed.
Materials and methods
Egg collecting
Eggs of D. minutus are mainly deposited into bamboo metaxylem vessels [1, 4, 6]. Collecting such eggs unharmed from bamboo pieces would be very difficult, and an alternative method was needed. Kartika and Yoshimura [9] and Baba and Ainara [10] reported techniques utilizing nutrient-containing filter paper to collect eggs of the powderpost beetle species Lyctus africanus and L. brunneus, respectively, and we adapted these techniques for D. minutus. Sheets of filter paper cut into 26 × 65 or 26 × 20 mm rectangles were soaked in an aqueous suspension containing 10% corn starch and 10% granulated sugar, and then dried at 60 °C for 1 h. Five-layered laminates of treated filter paper were formed by folding sheets of 26 × 65 mm four times or stacking five sheets of 26 × 20 mm. These laminates were fixed between two microscope slides with a string (Fig. 1). The laminates of filter paper were exposed to 10–20 adults of D. minutus inside Petri dishes and kept in an environmental chamber conditioned at 28 °C and 65% RH. Adults were obtained from laboratory strains reared on madake (Phyllostachys bambusoides) culms. The sexes of adults were not distinguished because adults do not have apparent morphological sexual characteristics [11]. Many of the adults bored into the laminates of filter paper after exposure and, usually within 2 days, the females began to oviposit along the bored tunnels (Fig. 2a) or at the edges of the laminates. The boring behavior into laminates was not reported for Lyctus species, which usually laid eggs at the edges [9, 10]. The eggs (Fig. 2b) were collected carefully using the tip of a writing brush, and the hatched larvae (Fig. 2c) were used for inoculation into bamboo pieces. Observations of 24 eggs showed that the average length and diameter were 0.84 ± 0.06 mm (mean ± SD) and 0.15 ± 0.01 mm, respectively, the average incubation period was 5.0 ± 0.8 days, and the average body length of newly hatched first instar larvae was 0.79 ± 0.04 mm.
Microscope photographs of eggs and a first instar larva collected from the laminates of filter paper. Note that photographs a–c do not show the same individuals. a Eggs (in ovals) laid between the layers of filter paper laminates along the tunnel bored by the adult beetle. b Egg removed from the filter paper. Anterior end upper. c First instar larva immediately after hatching. Anterior end upper
Preparation of bamboo specimens and inoculation of larvae
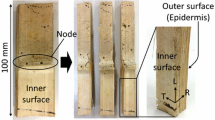
Air-dry internodes of madake culms [6–7 mm (R) thick] felled in June 2014 in Kyoto Prefecture, Japan, were split and cut into 14 pieces of 100 (L) × 20 (T) mm, which were used as the rearing medium. A hole with a depth of 5 mm was drilled longitudinally on one end surface of each piece with a 2.5-mm drill bit and the hole was extended by 5 mm with a push pin (Fig. 3). The shape of this hole was recorded by CT scanning prior to inoculation. In addition, longitudinally aligned holes with a depth of 1.5 mm (R) and a diameter of 1.5 mm were drilled on the outer surface (epidermis) of each piece with intervals of 5–25 mm as location references (Fig. 3). A first instar larva immediately after hatching was placed in the hole on the end surface of each piece using a writing brush, and the hole was closed by inserting a round bamboo stick [3 mm (L) × φ2.5 mm]. The bamboo pieces were kept vertically, with the inoculated end surface on the top, in the environmental chamber conditioned at 28 °C and 65% RH, in which the moisture content of the pieces was 11%.
X-ray CT scanning and CT data analysis
The inoculated bamboo pieces were scanned using a microfocus X-ray CT system (SMX-160CT-SV3S, Shimadzu Corp., Japan) with half-scan CT every 3–5 days. Four different scanning protocols with different resolutions (voxel sizes) were employed according to the larval body size. The scanning parameters, including the operation of the X-ray tube, for each protocol are shown in Table 1. Protocol I, with the highest resolution, was used when the larval body length was smaller than ca. 1 mm, and later, protocols with lower resolutions and larger field of view were used to trace the larvae that tunneled for longer distances. Regardless of the employed protocols, the larval body lengths were mostly within 35–60 pixels and the axes of the tunnel cross sections were mostly within 15–25 pixels. The volumetric data obtained from one scan consisted of 464–488 slices of 512 × 512 pixels. The scanning time during which the pieces were irradiated was 160 s.
2D tomograms from the volumetric data were used to measure the larval body length, tunnel length, and tunnel cross-sectional area using ImageJ 1.47v software (W. S. Rasband, National Institutes of Health, USA). The larval body was curved except in the prepupal stage, so the body length was measured by the segmented line tool. The length of the tunnels was measured using the aligned holes on the outer surfaces as location references. The tunnel volume was estimated by assuming that the cross section of the tunnel was elliptic and kept the same area in a scanning interval of 3–5 days. The major and minor axes of the cross section of the tunnel near the end were measured to calculate the cross-sectional area. The volume change from the previous scan was estimated by multiplying the cross-sectional area by the increase in tunnel length.
CT scans were continued after adult eclosion. The bamboo pieces containing adults were individually kept in glass bottles in the environmental chamber with no light source.
Results and discussion
Larval–pupal development and larval feeding
Of the 14 first instar larvae, three either died or were lost track of by the time of the first CT scanning. The other 11 individuals all successfully pupated and emerged into adults. The results and discussion are based on these 11 individuals.
Figure 4 shows the growth and developmental process of a typical individual from first instar to adult captured in CT images. In the CT images, the gray level of a pixel is an index of density; the brighter the pixel is, the denser the area. With the highest resolution (smallest voxel size) of 18 μm, silhouettes of the early stage larvae were visible in the CT images. The inoculated larvae tunneled into the parenchyma from near the tip of the inoculation holes made by the push pin. All of them bored downward, except one which bored upward at first but then reversed its direction after reaching the drilled part of the inoculation hole. The larvae bored linearly along the fibers most of the time. However, prior to pupation, three larvae created transverse tunnels in order to reach the surface and discharge frass from tiny holes; two larvae from the inner surface and one from the outer surface. This is the reason why, in Fig. 4, the tunnel was hollow after 66 days. Three other larvae bored slightly obliquely in the mature larval stages, one of which eventually reached the inner surface and discharged frass. Because each bamboo piece was inoculated with a single individual, no complex tunneling patterns were observed.
Growth and development of an individual from first instar to adult captured in CT images. Scanning Protocol I was used for the first and second CT images on the upper row, Protocol II and III for the third and fourth images, respectively, on the upper row, and Protocol IV for the images in the bottom row. Yellow squares in the illustration of the bamboo piece on the left of the figure represent the approximate position of the beetle at the noted time elapsed after hatching. Note that larval instars given for reference were determined based on the result of AE monitoring, which were not discussed in this paper
Figure 5 shows the time course of the body length of the 11 individuals from the first CT scan until adult eclosion. At first, the body lengths of all the larvae were smaller than 0.79 mm, the average body length of larvae immediately after hatching, suggesting that the larvae had shrunk. After the larvae had fully grown, they turned into prepupae and then pupated. However, the prepupal stage, lasting for approximately 1 day, was captured only for four larvae. The average body length in the final larval stage, including the prepupal stage if captured, was 3.53 ± 0.23 mm (mean ± SD). The average body lengths of pupae and adults were 3.42 ± 0.09 and 3.18 ± 0.17 mm, respectively. The average larval duration, calculated as the time from inoculation to the first observation of either the prepupal or pupal stage, was 61 ± 11 days. In this study, when discussing larval durations, the larvae were regarded as having pupated when the silhouettes of prepupae were captured. The total duration of larval and pupal stages, calculated as the time from inoculation to first observation of the adult stage, was 66 ± 11 days. The average pupal duration, therefore, was estimated to be 5 days. As suggested in our previous report [8], the CT images provided no clear evidence of ecdysis in the courses of larval development; findings regarding larval ecdysis and instars will be discussed in a separate report, in preparation, on acoustic emission (AE) monitoring of larval development, a study following the clarification of the relationship between the feeding and the generation of AE waves [12].
Figure 6 shows the time course of the tunnel length in the larval stage, until either the prepupal or pupal stage was first observed. Because the larvae filled their tunnels with frass as they extended their tunnels, the tunnel length represents the distance they moved. The “tunneling speed” increased as the larvae developed from the first to final instars, and prior to pupation the tunnel length reached 80.2 ± 4.8 mm. The average tunneling speed for the entire larval stage was 1.34 ± 0.20 mm/day.
Figure 7 shows the time course of the tunnel cross-sectional area, measured near the end of the tunnel where the area was largest, from the first CT scan to the prepupal or pupal stage. The cross-sectional area increased as the larvae grew and had a strong linear correlation with the square of the larval body length (y = 0.145x 2 + 0.05; R = 0.98). The final values of the cross-sectional area, 2.01 ± 0.13 mm2 on average, represented the cross-sectional area of the pupal chambers, whose diameter was approximately 1.6 mm.
Figure 8 shows the time course of the tunnel volume in the larval stage. The larvae bored and consumed 68.0 ± 7.0 mm3 of bamboo in the entire larval stage. At first, the volume consumed was small but it increased substantially as the larvae grew; the 90% of the total bamboo consumption was done in the latter 41% of the larval duration on average. This suggests that early detection and treatment can significantly reduce the extent of damage caused by the larvae. The correlations between total tunnel volume and final larval body length, pupal body length, and adult body length, respectively, were not especially high (R = 0.46, 0.76, and, 0.43, respectively), and only the correlation between total tunnel volume and pupal body length was significant (p < 0.01). It is possible that parts of tunnels, for example, those made to discharge frass or made as pupal chambers, were not utilized as nutrient sources, causing variation in the tunnel volume and, hence, lower correlations.
Using X-ray CT, larval attack of D. minutus was evaluated in terms of tunnel length and volume, which have not been reported previously for D. minutus except for our previous paper [8], and we consider this to be a unique feature of utilizing nondestructive X-ray CT scanning. With the aid of laminated filter paper to collect eggs, the tunnel length and volume bored for the entire larval period per individual, as well as the time changes of these, were revealed for the first time. In addition, the growing body size and developmental period were measured without exposing or removing the larvae. From these experiments, we have developed a methodology for the nondestructive evaluation of growth (body size increase), tunneling behavior, and bamboo consumption from first instar to pupation and adult eclosion using X-ray CT. This methodology can be further applied to clarify effects of environmental factors, such as temperature and humidity, on development and feeding. It can also be useful to nondestructively evaluate the efficacy of practical protection and extermination measures.
Discussion on larval duration
Irradiation, depending on the applied doses, can damage, sterilize, and kill living organisms and has been used to disinfest insects such as pests in stored foods. Irradiated insect larvae may suffer delayed development and may fail to molt, pupate, or emerge into adults [13, 14]. In the report in preparation on AE monitoring of the larval development, we will show that the average larval duration of eight un-irradiated individuals was 64.8 days (Watanabe et al., unpublished data). The larval duration of irradiated larvae in this study was not significantly different from that of un-irradiated larvae (p > 0.1; Student’s t test). In addition, all of the successfully inoculated larvae pupated and emerged without abnormality after repeated X-ray CT scans. Therefore, we consider that the X-ray irradiation employed during CT scans did not hinder the development of the larvae.
Several previous reports from outside of Japan describe the larval duration of D. minutus and are summarized here for reference. Plank [15] reported that, with monthly average temperatures of 26.0–26.8 °C, the larval duration was 41.4 ± 1.5 days (mean ± SE, n = 98). Garcia and Morrell [4] measured larval duration at different temperatures (15–30 °C) to determine the thermal thresholds and requirements, and at temperatures close to our experimental condition: 25, 28, and 30 °C, the larval duration was 51.7 ± 1.2 days (mean ± SE, n = 24), 46.3 ± 0.7 days (mean ± SE, n = 25), and 43.8 ± 0.5 days (mean ± SE, n = 26), respectively. According to Abood and Norhisham [5] and Norhisham et al. [6], the larval duration of individuals fed with cassava powder at 27 °C was 52.80 ± 0.31 days (mean ± SE, n = 50) and that of individuals reared on bamboo at 25 °C was 44.2 ± 0.3 days (mean ± SE, n = 20), respectively. Our value of larval duration found by CT scans, 61 ± 3 days (mean ± SE, n = 11), was longer than those reported previously, but these values cannot be simply compared because of underlying differences in many factors. As Garcia and Morrell [4] showed, temperature greatly influences larval development. However, temperature hardly explains the differences among these values because of the extremely low correlation (R = 0.04). Other possible factors may include regional and population differences and differences in species, nutrient contents, and moisture conditions of the employed rearing media. Some Japanese books describe the larval duration of D. minutus to be 20 days [3] or 20–40 days [2]; however, the methods of examination are not noted in these books and the validity of these values cannot be discussed.
The larval duration varied greatly from 49 to 81 days in this study. The final larval body length, pupal body length, and adult body length were not significantly correlated with larval duration (p > 0.1). This suggests that, although the variation in larval duration, and hence the variation in rate of development, may be inherent in D. minutus, each larva is capable of developing into an adult of a certain body size. Our results may also explain the simultaneous presence of larval and adult stages throughout the year.
Pre-mating adult feeding
Adult beetles started boring new tunnels within 3 days after eclosion. They made holes that were smaller than their body size on the inner surfaces of bamboo and discharged frass from these holes. In 8 ± 1 days (mean ± SD) after eclosion, they made exit holes on the inner surface, from which frass was also discharged. Even after making exit holes, the adults usually remained hidden inside the bamboo pieces. When the pupal chambers were not adjacent to the bamboo inner surface, the adults first bored obliquely to the fibers to reach the inner surface. Afterwards, most of the adults bored tunnels parallel to the fibers, except two individuals whose tunnels were oblique to the fibers by 43° or 68°. An example CT image of a tunnel created in this period is shown in Fig. 9. The changes in tunnel length and volume bored by the adult beetles were measured in the same manner as those of the larval tunnels. Adults extended tunnels at average rates of 2.64 ± 0.58 mm/day in length and 4.87 ± 1.10 mm3/day in volume until they first made exit holes. The adult tunnels were slightly narrower than the pupal chambers, with an average cross-sectional area of 1.86 mm2.
CT image capturing a tunnel created by Reifungsfrass of the same individual as shown in Fig. 4, obtained at 8 days after adult eclosion
The adult tunneling behavior described above corresponds to “Reifungsfrass”, a feeding behavior necessary for the maturation of newly emerged adults [1]. The pattern of tunneling in the longitudinal direction during this period has been reported previously [1, 2], and the result of our observation was consistent, though there were a few exceptions in this study. However, actual damage during Reifungsfrass was quantified for the first time.
Mature adults exit the culms and mate, and mated females re-enter bamboo culms and bore new tunnels in which to lay eggs [1,2,3,4]. It was reported that female adults tunnel transversely to the bamboo fibers in the process of oviposition [1, 2, 4]. However, details of the extent of damage they cause during the ovipositional period are unknown, and nondestructive analysis of the ovipositional behavior is a topic of subsequent research. It is also expected that deposited eggs can be visualized and counted using X-ray CT scanning.
Conclusions
Laminates of filter paper containing sugar and starch proved to be an effective artificial oviposition medium for D. minutus and facilitated monitoring from the first instar. The eggs were 0.84 ± 0.06 mm (mean ± SD) in length and hatched in 5.0 ± 0.8 days. Using X-ray CT scanning, the progression of larval body size and tunnel size was revealed and quantified. The results showed that the larvae grew to 3.53 ± 0.23 mm in body length and bored tunnels with a length of 80.2 ± 4.8 mm, consuming 68.0 ± 7.0 mm3 of bamboo per individual. The larval duration was 61 ± 11 days and the pupal duration was estimated to be 5 days. Damage caused by pre-mating adults during Reifungsfrass was also evaluated. The larval duration of individuals used in this study was longer than in previous reports, but this was not due to the development inhibition effects of irradiation. There was a large variation in larval duration, but it was suggested that body size was not dependent on larval duration. This methodology utilizing X-ray CT can be the basis for nondestructive evaluation of development and feeding for further investigation.
Change history
20 February 2018
The article Nondestructive evaluation of egg-to-adult development and feeding behavior of the bamboo powderpost beetle Dinoderus minutus using X-ray computed tomography, written by Hiroki Watanabe, Yoshiyuki Yanase and Yoshihisa Fujii, was originally published Online First without open access.
References
Wood Technological Association of Japan (1961) Mokuzai-hozon handbook (Wood protection handbook) (in Japanese). Shokodo, Tokyo
Yamano K (1969) Kenchiku-konchu-ki (Insect pests of buildings) (in Japanese). Sagami Shobo, Tokyo
The Society of House and Household Pests Science, Japan (1995) Kaoku-gaichu-jiten (Encyclopedia of house and household pests) (in Japanese). Inoue Shoin, Tokyo
Garcia CM, Morrell JJ (2009) Development of the powderpost beetle (Coleoptera: Bostrichidae) at constant temperatures. Environ Entomol 38:478–483
Abood F, Norhisham AR (2013) Larval development of the bamboo borer (Dinoderus minutus Fabricius) using individual rearing method. Pertanika J Trop Agric Sci 36:55–66
Norhisham AR, Faizah A, Zaidon A (2015) Effects of moisture content on the bamboo borer Dinoderus minutus. J Trop Forest Sci 27:334–341
Norhisham AR, Abood F, Rita M, Hakeem KR (2013) Effect of humidity on egg hatchability and reproductive biology of the bamboo borer (Dinoderus minutus Fabricius). SpringerPlus 2:9
Watanabe H, Yanase Y, Fujii Y (2015) Evaluation of larval growth process and bamboo consumption of the bamboo powder-post beetle Dinoderus minutus using X-ray computed tomography. J Wood Sci 61:171–177
Kartika T, Yoshimura T (2013) Nutritional quality of diet and fecundity in Lyctus africanus (Lesne). Procedia Environ Sci 17:97–104
Baba Y, Ainara K (2014) Development of control method for powder post beetle: examination of inducing oviposition method and evaluation of inhibition efficacy of pesticides for oviposition and hatching (in Japanese). Mokuzai Hozon (Wood Prot) 40:64–69
Abood F, Norhisham AR, Shahman M, Andy A (2010) Sexual identification of bamboo borer Dinoderus minutus (Fabricius) (Coleoptera: Bostrychidae). Malays For 73:1–6
Watanabe H, Yanase Y, Fujii Y (2016) Relationship between the movements of the mouthparts of the bamboo powder-post beetle Dinoderus minutus and the generation of acoustic emission. J Wood Sci 62:85–92
Johnson J, Marcotte M (1999) Irradiation control of insect pests of dried fruits and walnuts. Food Technol 53:46–51
Follett PA (2004) Irradiation to control insects in fruits and vegetables for export from Hawaii. Radiat Phys Chem 71:161–164
Plank HK (1948) Biology of the bamboo powder-post beetle in Puerto Rico. Bulletin No. 44, Federal Experiment Station in Puerto Rico, Mayaguez
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (nos. 25242032, 26450229, and 17J04018) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10086-018-1707-y.
About this article
Cite this article
Watanabe, H., Yanase, Y. & Fujii, Y. Nondestructive evaluation of egg-to-adult development and feeding behavior of the bamboo powderpost beetle Dinoderus minutus using X-ray computed tomography. J Wood Sci 63, 506–513 (2017). https://doi.org/10.1007/s10086-017-1642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-017-1642-3