Abstract
The decay risk of airborne wood-decay fungi in the same volume of air was investigated by using an air sampler over the course of a year at three different sampling sites. Japanese cedar disks measuring 7.8 cm in diameter and about 3 mm in thickness, and with a moisture content of about 100 % were placed in a “BIOSAMP” air sampler and then exposed to 1000 l of air in the northern, central, and southwest parts of Japan. The exposed disks were incubated for 20 weeks in a damp container maintained at 26 ± 2 °C and degraded by fungi trapped on the disks. The decay risk was calculated from the mass loss during incubation, and the factors affecting the said risk were explored. The results showed that sampling sites apparently do not affect decay risk, even though the Scheffer’s climate indexes of the sites were quite different. The relation between the sampling month and decay risk reveals that decay risk remains virtually the same year-round. Relative humidity on a sampling day is one of the key factors affecting decay risk in sampling conducted at the central or southwest site. In contrast, no weather factors influenced decay risk at the northern sampling site.
Similar content being viewed by others

Introduction
Since harvested wood products have been defined as a carbon sink [1, 2], it is very important to prolong the service life of wooden products and delay their emissions of carbon dioxide gas. One possible method of retarding gas emissions is through wood protection [3], and many investigative studies have been conducted on preservatives and chemical treatments [4–6].
Research on the factors that accelerate carbon dioxide gas emissions from wooden structures is also important and has revealed that one of the most important biological factors in accelerating emissions is wood-decay fungi [7, 8]. The decay risk of wooden products was consequently investigated through various conventional testing methods, such as field tests [9–11], the lap joint test, L-joint test and double-layer test [12], and is thought to be predicted by Sheffer’s climate index [13] in the case of outdoor aboveground wood products. In addition to these conventional studies and new study using potato dextrose agar plates [14], we developed a novel method of assessing the decay risk of airborne wood-decay fungi in a certain amount of air by using an air sampler and wood disks [15, 16].
In our previous paper [15], we employed a novel method of assessing decay risk in the same volume of air and concluded that relative humidity on a given sampling day influenced the decay risk. The study was not complete, however, as the previous study lacked data on the winter season. Moreover, the study was only conducted at one sampling site located in central Japan. Therefore, whether the above relation applies to other sampling sites remains unclear.
To confirm the question posed above, we conducted similar experiments at different sites whose climate indexes are quite different. This paper examines the decay risk of airborne wood-decay fungi at these sites during the course of an entire year. It also discusses the weather factors affecting decay risk.
Materials and methods
Preparation of wood specimens
Wood specimens were prepared using the method employed in the previous study [15]. Japanese cedar (Cryptomeria japonica) disks about 3 mm thick were dried at 60 °C for 1 day and then weighed to determine their initial mass (M 0). Disks were randomly placed in individual Petri dishes and then into individual sealed bags for gas sterilization. Gas sterilization was carried out in ethylene oxide gas sterilizers (Model YS-A-C64E, Yuyama Co. Ltd., Osaka). Each disk in a sterilized sealed bag was randomly sent to three sampling sites.
Sampling and incubation procedure
Air sampling was conducted at the three sampling sites listed in Table 1. The sampling points at the sampling sites were chosen to have similar surrounding environments, and air at each site was sampled on a sunny or a cloudy day, but not on a rainy day.
A plate containing a Japanese cedar disk kept in a sterilized sealed bag was removed from the bag at each site just before air sampling. Six mL of sterilized deionized water was also added aseptically to each disk. The “BIOSAMP” air sampler (Midori Anzen Co. Ltd., Tokyo) was used to conduct air sampling. The sampling procedure followed the product manual except for replacing the agar medium for damp Japanese cedar disks. Each disk exposed to 1000 l of air at about 100 cm in height was kept in a plastic plate and incubated in a damp container at 26 ± 2 °C. After incubation for 20 weeks, each disk was dried at 60 °C for 2 days. Then the disk mass after incubation (M 1) was measured. The mass loss during the incubation period was calculated from M 0 and M 1.
Collection of weather data
Weather data for the N-site and that for the C-site were collected by the MC-2100 weather monitoring system (Koshin Denki Kogyo co. Ltd., Tokyo) and the C-CR1000 weather monitoring system (CLIMATEC Inc., Tokyo), respectively. Both systems were set at about a few hundred meters away from each sampling site. Weather data for the SW-site was obtained from Japan’s Meteorological Agency (as monitored at about 3.5 km west of the SW-site).
Scheffer’s climate index [13] at each site was calculated according to the following equation:
where T denotes the mean monthly temperature (°F) and D the mean number of days in the month with 0.01 in. or more of precipitation.
Statistical analysis
All statistical analyses were conducted using JMP 9.0 software (SAS Institute, Cary, North Carolina, USA) with a significance level set at 0.05 [17].
Results and discussion
Decay risk and sampling sites
Figure 1 shows the mean monthly air temperature at the three sampling sites during the sampling period from May 2010 to May 2011. As expected from the latitude of each site listed in Table 1, the air temperature of each sampling site decreases in line with higher site latitude. For instance, the mean monthly air temperature at the N-site was about 10 °C lower than at the other sites. Figure 2 plots the amount of precipitation at each site. Although the monthly data varied widely, particularly at the C-site and SW-site, the total precipitation at each site also decreased in line with the sampling site latitude.
Since the decay risk of outdoor aboveground wood products is known to be in accordance with Scheffer’s climate index [18], the climate index of each sampling site is calculated (Table 2). According to Scheffer’s climate index, the decay risk must increase in line with lower sampling site latitude.
To compare our results with the decay risk predicted by Scheffer’s climate index, we first investigated the relation between the sampling sites and the mass loss caused by airborne wood-decay fungi. Figure 3 shows the distributions of mass loss at the three sampling sites. The figure suggests no differences among the mass loss at each sampling site, even though the climate indexes of the three sites are quite different. The non-parametric Steel–Dwass test revealed no significant differences among the mass loss at each sampling site. Our experimental results clearly indicated about the same level of decay risk caused by airborne fungi at all three sampling sites.
Perhaps, the reason why the experimental results of this study differ from those predicted by Scheffer’s climate indexes may be the temperatures at which the wood specimens were incubated. Scheffer’s index is an equation used to estimate the decay risk of outdoor aboveground wood products. Therefore, the index must include the effect of outdoor temperature in the equation. In contrast, we set the temperature to 26 °C during the incubation period because we intended to estimate the decay risk of airborne wood-decay fungi in 1000 l of air. Thus, Scheffer’s climate index was considered unsuitable for predicting the decay risk of wood products placed in an environment where the products’ ambient temperature did not change with the outdoor temperature. Timber frames covered with insulating materials may be one wood product whose temperature is not affected by outdoor temperature. Scheffer’s climate index must preferably be used to define the decay risk of building materials outside of insulators, but not inside them.
Sampling month and decay risk
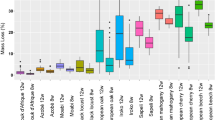
In the previous paper [15], we reported that decay risk was not affected by the sampling month. However, we reached this conclusion based on sampling data collected from June to December in 2008. To confirm whether the conclusion can be applied to the winter season, air sampling in this study was conducted over the course of an entire year. Figure 4 shows the relation between the sampling month and the mass loss at each site. The months of winter showed relatively low decay risk, but no differences were observed in the months of other seasons. Nonparametric comparisons for all pairs using the Steel–Dwass test was applied to investigate whether any significant difference existed in terms of the decay risk in each month. Figure 4 also shows the results. At all three sampling sites, significantly lower decay risk (p < 0.05) was observed in 1 month during winter, but no significant differences were observed among the other months. We therefore conclude that at present, the decay risk is virtually the same in each month at each area, even though the risk drops during 1 month in winter.
Relation between sampling month and mass loss at three different sites. The vertical lines within the boxes represent the median sample values; the ends of the boxes represent the 75th and 25th quantiles. The upper and the bottom lines indicate the values calculated as follows: upper line, 3rd quartile + 1.5 × (75th quantile − 25th quantile); lower line, 1st quartile − 1.5 × (75th quantile − 25th quantile). Different letters indicate significant difference at p < 0.05 by Steel–Dwass’s test
Figure 4 also shows another fact worthy of mention. As listed in Table 1 and shown in Fig. 1, the N-site is located in northern Japan, and the mean air temperature during the winter season drops below 0 °C. Therefore, any rainfall at this site in winter becomes snow, which remains on the ground until spring. During the sampling period from January to March 2011, the snow was about 30–90 cm deep at this site. On the other hand, decay risk at the N-site does not remain zero throughout the year. Moreover, higher mass loss was observed in several wood disks exposed to air in winter. It is reported that many wood-decay fungi preferentially produce spores between zero to 10 °C [19] and release them to the environment [20, 21]. Some wood-decay fungi at N-site also release spores even under such conditions as ambient air temperature around the fruit body near 0 °C, though the ground is covered with thick snow.
Effect of weather factors
In the previous study, the decay risk of airborne wood-decay fungi was confirmed as being correlated with weather factors that indicated sampling was done on a cloudy day. Mean relative humidity and lowest relative humidity on the sampling day are among the most relevant factors in assessing decay risk. This fact looks acceptable because spore germinations are affected by the wood moisture content that becomes higher in line with higher relative humidity [22–24]. Therefore, fungi must release more spores on a cloudy day than a shiny day.
To confirm this relation, retests were done at all three sites as previously mentioned (Table 1). Figure 5 shows the relation between the mass loss of the disks and the mean relative humidity on the sampling day at each sampling site. Regression lines are also given in the figure. As shown in the figure, the relation between mass loss and relative humidity is not the same at all three sampling sites. At the C-site and the SW-site, the relation is the same as that observed in the previous study, that is, a positive correlation exists between mass loss and mean relative humidity on the sampling day (p < 0.05). Contrary to this relation, the mass loss of disks exposed at the N-site was not affected by the mean relative humidity on the sampling day. This inconsistency may be partially due to fungi that caused mass loss above 1000 mg during the 20-week incubation period. Many such fungi appeared on a day of low relative humidity. The DNA sequences of these fungi are now being analyzed and will be discussed in our next paper.
Relation between relative humidity and decay risk. Each solid line indicates the linear least square fit of mass loss against relative humidity given by the following equation: N-site, mass loss = 0.207 × relative humidity + 342; C-site, mass loss = 6.93 × relative humidity – 142; SW-site, mass loss = 6.02 × relative humidity − 98.3
Table 3 summarizes the relation between decay risk and weather factors. These results suggest that weather factors affecting decay risk are not equal among the different sampling sites.
The decay risk by airborne wood-decay fungi is affected by relative humidity, the amount of solar radiation, and duration of sunshine at the C-site, and by temperature, relative humidity, and atmospheric pressure at the SW-site. Positive and negative signs of the correlation coefficient of these factors suggest that decay risk increases on humid and cloudy days at the C-site, and on warm and humid days with low atmospheric pressure at the SW-site.
In contrast to these warmer sites, the N-site shows that its decay risk is not influenced by any weather factors. It will be interesting to reveal whether any correlation exists at other high-latitude areas similar to the N-site.
References
UN FCCC (2011) Decision -/CMP.7 -Land use, land-use change and forestry- http://unfccc.int/files/meetings/durban_nov_2011/decisions/application/pdf/awgkp_lulucf.pdf. Accessed May 17, 2013
Tsunetsugu Y, Tonosaki M (2010) Quantitative estimation of carbon removal effects due to wood utilization up to 2050 in Japan: effects from carbon storage and substitution of fossil fuels by harvested wood products. J Wood Sci 56:339–344
Deroubaix G (2008) Wood protection, a tool for climate change mitigation? International Research Group on Wood Protection, Document No. 08-50257
Hermawan A, Nakahara T, Sakagami H, Fujimoto N, Uchikura K (2013) Performance of Sugi lamina impregnated with low-molecular weight phenolic resin. J Wood Sci. doi:10.1007/s10086-013-1338-2
Ali Ahmed S, Sehlstedt-Persson M, Hansson L, Morén T (2013) Evaluation of preservative distribution in thermally modified European aspen and birch boards using computed tomography and scanning electron microscopy. J Wood Sci 59:57–66
Kim G-H, Hwang W-J, Yoshimura T, Imamura Y (2010) Laboratory evaluation of the termiticidal efficacy of copper HDO. J Wood Sci 56:166–168
Hunt GM, Garratt GA (1967) Agencies of wood deterioration. In: Wood Preservation, McGraw-Hill, New York, pp 21–46
Zabel RA, Morrell JJ (1992) Wood deterioration agents. In: Wood microbiology—decay and its prevention. Academic Press, California, pp 21–51
Brischike C, Rapp AO (2008) Dose–response relationships between wood moisture content, wood temperature and fungal decay determined for 23 European field test sites. Wood Sci Technol 42:507–518
Brischike C, Rapp AO (2008) Influence of wood moisture content and wood temperature on fungal decay in the field: observation in different micro-climates. Wood Sci Technol 42:663–677
Sakai H (2009) Fungal resistance test for non-treated and treated wood in the field (in Japanese). J Soc Mater Sci 58:271–279
Råberg U, Edlund ML, Terziev N, Land CJ (2005) Testing and evaluation of natural durability of wood in above ground conditions in Europe—an overview. J Wood Sci 51:429–440
Scheffer TC (1971) A climate index for estimating potential for decay in wood structures above ground. Forest Prod J 21(10):25–31
Toyoumi A, Horisawa S, Yoshimura T, Doi S, Imamura Y (2010) The effect of different foundation systems on the fungal flora in the crawl space of a new wooden Japanese house. Build Environ 45:1054–1060
Momohara I, Ota Y, Nishimura T (2010) Assessment of decay risk of airborne wood-decay fungi. J Wood Sci 56:250–255
Momohara I, Ota Y, Sotome K, Nishimura T (2012) Assessment of decay risk of airborne wood-decay fungi II: relation between isolated fungi and decay risk. J Wood Sci 58:174–179
JMP Introductory Guide.9.0.2 (2011) SAS Institute Inc., Cary
Morris P, Wang J (2011) Scheffer index as preferred method to define decay risk zones for above ground wood in building codes. Int Wood Prod J 2:67–70
Nuss I (1975) Zur Ökologie der Porlinge. Bibliotheca Mycologica 45: Abb. 3, 15–16
McCracken FI (1978) Spore release of some decay fungi of southern hardwoods. Can J Botany 56:426–431
McCracken FI (1987) Observations on the spore release of Paxillus panuoides. Grana 26:174–176
Griffin DM (1977) Water potential and wood-decay fungi. Ann Rev Phytopathol 15:319–329
Viitanen H (1994) Factors affecting the development of biodeterioration in wooden constructions. Mater Struct 27:483–493
Carll CG, Highley TL (1999) Decay of wood and wood-based products above ground in buildings. J Testing Eval 27:150–158
Acknowledgments
The authors would like to thank Dr. Tsutomu Hattori for his useful advice on fungal ecology. This work was partly supported by Grant-in-Aid for Scientific Research (B) and (A). We are grateful to Ms. Yuri Inami, Ms. Junko Kamigata, and Ms. Naoko Kawachi for their considerable contribution to this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Momohara, I., Ota, Y., Yamaguchi, T. et al. Assessment of the decay risk of airborne wood-decay fungi III: decay risks at different sampling sites. J Wood Sci 59, 442–447 (2013). https://doi.org/10.1007/s10086-013-1355-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-013-1355-1