Abstract
For making efficient use of waste wood ash emitted from wood biomass plant, the wood and wood ash-based hydroxyapatite (HAp) composite was produced and their flammability characterization was studied by thermogravimetric (DTA-TG) analysis, oxygen index (OI) measurement and cone calorimeter test. The results show that the exothermic and weight loss peaks in DTA-TG combustion profiles due to their significant thermal decomposition were weakened by the HAp agent impregnation. In addition, the OI value of HAp composites was increased by the HAp combining and the OI showed a correlation with the HAp contents. Also, the cone calorimeter study revealed that the heat release rates were decreased with increasing amount of HAp injection and accordingly their total heat release has an inverse relationship to the HAp contents. These results indicate that the treatment with wood ash-based HAp agents can enhance the flame retardancy of the treated woods.
Similar content being viewed by others
Introduction
For economic and environmental concerns, wood biomass has attracted a grate deal of attention as a substitute of fossil fuel in the past decades. In Japan, approximately 5 million tons of waste woods were produced annually from lumber sawing process [1] and also about 4.7 million tons of construction waste woods were produced in 2005 [2]. Among them, around 20–30 % was utilized as biomass fuel for energy production [1, 2]. Burning of this wood fuel generates a heat and/or electrical power at biomass-combustion plants, but at the same time enormous quantities of wood ash are excreted as waste byproducts. Since the ash content of wood is a few percent, the amount of ash emission is estimated to have reached several tens of thousands of tons each year in Japan. Moreover, given the future increased use of unallocated forest resources as wood biomass fuel, the wood ash emission is expected to increase more than ever.
At present, the wood ash is mostly treated as industrial waste and high disposal cost of them has pressed a management of biomass plants. The problem related to the disposal of wood ash is one of the most crucial factors that have been detrimental to the spread of woody biomass utilization; therefore, the beneficial reuses of the wood ash directly lead to the zero-emission use of wood biomass fuel. The ash recycling for practical applications contributes to the expanding of the wood biomass utilization, and until now, many developments have been carried out to produce salable products by reusing of them. Wood ash is reusable for horticultural fertilizer and raw material of concrete [3]. Also in recent study, new innovative attempts have been made to manufacture a composite board by combining the ash with wood particles and some adhesives [4]. In our study, the opportunity of another new use application method of the wood ash has been explored as a source of wood-inorganic mineral composites.
Wood ash, in other words the combustion residue of wood fuels, is mainly composed of inorganic components with unburned carbon. The first motivation of our study is to examine whether the wood ash could be put back into timbers again within the space of wood cells for the purpose of making the wood-inorganic mineral composites. In the previous studies, several wood-inorganic compound complexes have been developed with chemical agents to enhance the fire and decay resistances of the wood [5–8]. We, therefore, intend to produce a new composite material by combining the wood ash within woods. However, the ash does not fit in so easily with woods because it has high alkaline and hence causes striking discoloration of the combined woods. So in this study, the wood ash was preliminarily converted into hydroxyapatite (HAp; Ca10 (PO4)6 (OH)2) solution by a reaction with phosphoric acid, and then it was penetrated and fixed into woods to make the HAp-wood composites.
The HAp is well known as eco-friendly biomaterials and widely applied for industrial products, e.g., dental powder, bone-repairing or bone-replacing material. Since the HAp is water-insoluble material, it has a potential to be used as a fixative in wood preservative formulations. In the previous studies of wood-HAp combining, the HAp was used as fixatives of the antibacterial metal ions within woods [9], and it was also synthesized and fixed on the surface of modified bamboo with the phosphorylation methods [10]. Moreover, it is noteworthy that the HAp contains a phosphoric component like some kinds of fireproof agents; therefore, by combining with the HAp, the treated woods would improve their fire retardance. So in this study, the combustion behavior of the HAp-wood composites was examined using a thermogravimetric analyzer, an oxygen index flammability tester and a corn calorimeter to evaluate the effects of the wood ash-based HAp agent on the fire-retarding properties of the HAp-wood composites.
Materials and methods
HAp treating agent formulations
Wood ash used in this experiment was obtained from a wood biomass power facility (Wood energy cooperative, Miyazaki, Japan). The sampling of the ash was performed on 25 August 2007. The ash sample was fly ash mainly produced by a burning of barks and mill ends of Japanese cedar (Cryptomeria japonica) at around 600 °C and it was recovered by an electrical dust collector. Their chemical and mineralogical compositions were reported in a previous study [11]. The major element compositions were carbon (36–42 wt%), calcium (29–35 wt%), potassium (11–21 wt%) and sulfur (3.8–4.5 wt%) estimated as oxidized compounds except for carbon, and the major inorganic mineral components of the ash sample were calcium compounds, such as calcium oxide (CaO), calcium carbonate (CaCO3), calcium hydroxide (Ca(OH)2) and mineral dolomite (CaMg(CO3)2), as well as the non-calcareous salt, potassium chloride (KCl).
The procedures for formulating the wood ash-based HAp agent are following the report of a preceding trial [12]. Initially, 200 g of the wood ash was dissolved in 2 L of 1 M acetic acid and then the solution was filtered to gain the Ca source solution. Also, an aqueous sodium dihydrogen phosphate NaH2PO4 solutions were prepared; 72 g of NaH2PO4 (Wako Pure Chemical Industries Co., Ltd., Tokyo, Japan) was resolved in 2 L water and then the NaH2PO4 solution was alkalized by adding granular NaOH reagent 30 g. After that, the Ca solution from acidizing wood ash was added to alkaline NaH2PO4 solution in a glass vessel with magnetic stirring under the room temperature and accordingly, the milky gel suspension of amorphous HAp with around pH 7 was gained. The obtained precipitate solutions were adjusted to have a Ca/P ratio equal to 1.67 (stoichiometric ratio of HAp) in consideration of the Ca quantity of the treated wood ash. The previous study [12] has reported that the HAp microcrystal generation was confirmed on treated woods with the above HAp suspension from a reflection electron imaging and powdered X-ray diffraction analysis, and other possible by-product salts, e.g., calcium acetate were not detected because the conceivable by-product salts were water-soluble and accordingly the water-insoluble HAp microcrystals dominated on the treated woods. Through the repeated operation of evaporation to dryness of the HAp solution, followed by a flushing with water of the evaporation residue, the HAp yield was evaluated around 50 % on the amount of wood ash in weight.
Treatment of wood specimens with HAp suspension
The wood specimens 50 × 30 × 4 mm (L, R, T) from edge-grained sliced veneers of Japanese cedar and Japanese cypress (Chamaecyparis obtusa) sapwoods were prepared for the fire performance evaluation with a thermogravimetric analyzer and an oxygen index flammability tester. After oven-drying at 60 °C for 72 h, the wood specimens were soaked into the HAp suspension under evacuating by rotary pump at 50 torr (6.67 Pa) for 30 min, and subsequently placed in a pressurized air at 1.1 MPa for 24 h. To measure the HAp uptake of the wood specimens, the treated specimens were conditioned after the HAp impregnation for 24 h and then oven-dried again at 60 °C for 72 h.
Also for evaluating the fire-retarding properties with a corn calorimeter, the test specimens 99 × 99 × 15 mm (L, R, T) were prepared from boards of Japanese cedar and Japanese cypress. After oven-drying at 60 °C for 72 h, the specimens were treated with HAp solution in three ways based on the previous cone calorimeter studies [13–15]: (1) The HAp suspension was simply spread over the surface of the specimens to coat them, and then the specimens were cured at room temperature for 4 weeks. (2) The specimens were soaked into the 100 °C boiling water for 2 h, and then soaked into the HAp suspension for 24 h under atmospheric room condition at 20 °C. (3) The specimens were soaked into the HAp suspension and placed in a vacuum at 50 torr (6.67 Pa) for 30 min, subjected to pressurized air at 1.1 MPa for 24 h. After the impregnation processes (2) and (3), the specimens were removed from the HAp solution and dried under atmospheric conditions for 4 weeks.
After these HAp-coating or impregnating processes, the specimens were oven-dried again at 60 °C for 1 week until the specimens mass stayed constant and the dry HAp retentions of all HAp-treated specimens were given in Table 1. The HAp-treated specimens and other untreated ones were stored in a temperature/humidity-controlled room at 20 °C and RH of 65 % for 4 weeks before the combustion tests.
Thermal property and fire performance evaluation
Thermogravimetric analyze
The powdered and sifted samples of the HAp composites through 0.5 mm screen were studied with a thermogravimetric analyzer (TGA-60, Shimadzu Co., Ltd., Kyoto, Japan) and a differential thermal analyzer (DTA-60, Shimadzu Co., Ltd., Kyoto, Japan) in open atmosphere with Alumina (α-Al2O3) powder as a reference material. The temperature was raised from 30 to 600 °C at the heating rate of 20 °C/min, with a flow of dried air (100 mL/min).
Oxygen index flammability test
The oxygen index test was conducted according to JIS K7201-2 [16] using an oxygen index flammability tester (ON-1, Suga test instruments Co., Ltd., Japan). This test measures the minimum concentration of oxygen (i.e., oxygen index, or limiting oxygen index: OI) in a flowing mixture of oxygen and nitrogen that is required to maintain flaming combustion of specimen. The specimen is ignited in the glass holder with the mixed flow of oxygen and nitrogen so the entire top tip of them is burning like a candle, and if the specimen burns for at least 3 min after the igniter is removed, or if the specimen burns down 50 mm, the oxygen concentration is judged above the oxygen index (OI). Instead, if the specimen stops flaming before those criteria, the oxygen concentration is below the OI. The procedure is repeated with upper and lower oxygen concentrations by 0.5 % until the lowest concentration of oxygen that will satisfy the criteria is determined. The trial was conducted on the 5 groups of specimens with the same amount of the HAp injection from Japanese cedar and Japanese cypress, respectively.
Corn calorimeter test
The cone calorimeter (Toyoseiki, Japan) was also used for the fire-retardant experiment of the HAp composites at Kumamoto University, Japan. The specimen was wrapped in aluminum foil and exposed horizontally to an external heat flux of 50 kW/m2. A spark ignite was applied to ignite the specimens. During the test, oxygen concentration of the exhaust gas and sample weight was measured every 2 s, and the heat release rate (HRR) and total heat release (THR) were obtained. The corn calorimeter tests were conducted on two samples from each group of HAp-treated specimens (HA-0 to HA-3, Table 1).
Results and discussion
Thermogravimetric analysis
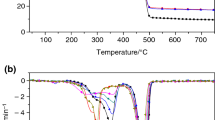
The results of the DTA and TG measurements are shown in Fig. 1. The untreated wood samples of Japanese cedar and Japanese cypress undergo two degradation steps, the first exothermic peak appeared at around 360 °C and the subsequent peak occurred at 480–490 °C due to their glowing. Their weight loss started gradually at around 270 °C and rapidly occurs at the exothermic peak temperatures. The similar thermal characteristics of Japanese cedar and Japanese cypress were reported in the previous study [17]. Meanwhile, in the case of HAp composites, it can be found that the HAp composite shows higher decomposition rate in TG curve up to around 300 °C with their exotherm starting, but subsequent exothermic peaks were weakened compared with the untreated samples and as a whole, exothermal and weight loss profiles of the HAp composites became smoother than those of the untreated samples. Also, the weakened exothermic peak temperatures of the HAp composites were shifted to the 20–30 °C lower in relation to those from the untreated ones. The similar changes in DTA-TG thermal profiles were reported in the previous studies especially intended for the wood treatment with phosphoric agents: e.g., fire-retardant-treated woods with several phosphoric agents [18] and cellulose crystals treated with phosphoric acid [19]; therefore, it implied that the injected HAp solution contributed to the thermogravimetric change of the treated woods as a phosphoric component carrier.
OI measurement
The results of the OI measurements are shown in Fig. 2. The OI values of untreated woods from Japanese cedar and Japanese cypress were 27.0 and 25.5, respectively, and the similar values were reported in previous study [20]. Once the untreated samples ignited at OI condition, they burned with a red flame and smoke. In contrast, the HAp-injected specimens were difficult to keep burning without raising the oxygen concentration and the HAp composites burned at around higher OI values with a small flame and less smoke. It can be observed from Fig. 2 that the OI values of the HAp composites with 40–60 kg/m3 HAp contents were raised to around 40–50 %. These OI values trended to be raised with increasing amount of the HAp injections, and there was a high correlation between the two. These results indicate that the injection of HAp solution can increase the flame retardancy of the treated woods.
Cone calorimeter study
Figures 3 and 4 show the heat release rate (HRR) curves of the HAp composites with Japanese cedar and Japanese cypress, respectively. The HRRs of all analyzed samples (HA-0 to HA-3) show two separate peaks during their combustion which indicate the gradual burning after the initial charred layers were formed. Although the second HRR peak risings were basically dependent on the experimental condition, e.g., thickness of test specimens and their adiabatic condition, the second HRR peaks were detected in this measurement as with the previous reports on the cone calorimeter studies of chemically treated woods [13–15].
Heat release rate (HRR) curves of HAp composites with Japanese cedar. See Table 1 for symbols HA-0 to HA-3
Heat release rate (HRR) curves of HAp composites with Japanese cypress. See Table 1 for symbols HA-0 to HA-3
In view of the HRR curves according to heating time, a first sharp HRR peaks appeared at the range up until 60 s, and at this time range, the peak HRR values of the HAp composites (HA-1 to HA-3) were slightly declined compared with those of the control (HA-0). Also over the following period from 60 to 300 s between two heat release peaks, the HRRs tend to be decreased with increasing amounts of the HAp contents and the subsequent rising of second HRR peaks tends to be delayed with the HAp increasing. These results reflected a parallel correlation between the mean heat release rates over the period to 60 and 300 s combustion (HRR60 and HRR300 shown in Table 2) and the amount of HAp contents.
Figure 5 shows the total heat release (THR) at lapse of 600 s burning. The THRs were also decreased with increased amount of the HAp injection in a reflection of HRRs reduction, and the declining rate of the THRs is similar between the HAp composites with Japanese cedar and those with Japanese cypress. These results indicate that the HAp treatment contributes to decrease the flammability of the treated woods, and these results from the cone calorimeter study are consistent with those from the OI measurement.
Total heat release (THR) at lapse of 600 s combustion from HAp composites with Japanese cedar (open circles) and Japanese cypress (filled circles). See Table 1 for symbols HA-0 to HA-3
Conclusions
It has been found that the wood ash-based amorphous HAp suspension has flame retardant effect on the HAp-treated woods. The HAp produces characteristic alterations as phosphoric agents in the thermogravimetric properties of the HAp-treated woods; the exothermic and weight loss peaks due to their significant thermal decomposition were weakened and peak temperatures were shifted to the lower. The OI measurements indicate that with the increase in the amount of HAp injection, it was becoming difficult to keep the combustion of the HAp composites without raising the oxygen concentration. Also in cone calorimeter study, the HRRs and THRs of the HAp composites tend to be decreased and the second HRR peak risings due to their gradual burning after the initial charred layers tend to be delayed with increasing amounts of the HAp contents. These results indicate that the HAp treatment contributes to decrease the flammability of the treated woods.
References
Ministry of Agriculture, Forestry and Fisheries, Japan (2007) Report on supply and demand of lumber 2005 (in Japanese), pp 58–59
Ministry of Land, Infrastructure, Transport and Tourism, Japan (2006) Report on the survey of construction byproduct recycling 2005 (in Japanese)
Udoeyo FF, Inyang H, Young DT, Oparadu EE (2006) Potential of wood waste ash as an additive in concrete. J Mater Civ Eng 18:605–611
Milena P, Reto P, Frederic P (2008) Wood ash as a resource for the production of innovative composite materials. 10th world conference on timber engineering, June 2–5, 2008, Japan
Imamura Y (1989) Development of unburnable woods “WIC” (in Japanese). J Soc Mater Sci Jpn 38:1224–1225
Furuno T, Uehara T, Jodai S (1991) Combinations of wood and silicate I—Impregnation by water glass and applications of aluminum sulfate and calcium chloride as reactants. Mokuzai Gakkaishi 37:462–472
Miyafuji H, Saka S (1997) Fire-resisting properties in several TiO2 wood-inorganic composites and their topochemistry. Wood Sci Technol 31:449–455
Ahn SH, Oh SC, Choi IG, Kim HY, Yang I (2008) Efficacy of wood preservatives formulated from okara with copper and/or boron salts. J Wood Sci 54:495–501
Yamaguchi H, Itou S, Matsunaga H (2003) Preservative ability of wood with hydroxyapatite with a part of Ca substituted by antimicrobial metals (in Japanese). Bokin Bobai 31:69–76
Li S, Liu Q, Wijn J, Wolke J, Zhou B, Groot K (1997) In vitro apatite formation on phosphorylated bamboo. J Mater Sci Mater Med 8:543–549
Akaki T, Fujimoto H, Asano R, Kashiwazaki K (2007) Inorganic components and their chemical forms in wood bark ash (in Japanese). Proceedings of the 14th annual meeting of the Japan Wood Research Society Kyushu branch, November 8–9, 2007, Japan, pp 31–32
Akaki T (2010) Development of hydroxyapatite-wood composites by reusing of wood ashes. The Internal Forestry Review of XXII IUFRO World Congress, August 23–28, 2010, Korea, pp 274
Harada T, Uesugi S (1996) Combustion properties of wood treated with chemicals in the cone calorimeter (in Japanese). Mokuzai Hozon 22:262–271
Kawarasaki M, Hiradate R, Yoshida S, Takizawa K, Okano A (2007) Investigation of quasi-noncombustible plywood made from trees grown in Hokkaido (in Japanese). J Hokkaido For Prod Res Inst 21:1–8
Kikuchi S, Maeda S (2007) Effects of fire retardant chemicals and retention on heat release rate of wood (in Japanese). Mokuzai Gakkaishi 53:276–282
JIS K7201–2 (2007) Plastics-determination of burning behaviour by oxygen index-Part 2: ambient-temperature test (in Japanese). JSA, Tokyo
Arima T (1973) Differential scanning calorimetry of wood and wood components I (in Japanese). Mokuzai Gakkaishi 19:435–442
Arima T (1974) Thermogravimetry and differential scanning calorimetry of wood veneers treated with flame retardants (in Japanese). Annual report of building research institute, pp 1–8
Kumagai Y, Ohuchi T, Nagasawa C, Ono M (1974) Effects of phosphoric acid on the pyrolysis of cellulose (in Japanese). Mokuzai Gakkaishi 20:381–387
Yoshimura M, Miwa A (1980) Oxygen index for wood in burning test II, Determination in JISK7201 method (in Japanese). Mokuzai Gakkaishi 26:287–292
Acknowledgments
This work was financially supported by Grant-in-Aid for Scientific Research (Challenging Exploratory Research, No. 23658150) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Akaki, T., Maehara, H. & Tooyama, M. Development of wood and wood ash-based hydroxyapatite composites and their fire-retarding properties. J Wood Sci 58, 532–537 (2012). https://doi.org/10.1007/s10086-012-1276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-012-1276-4