Abstract
We investigated morphological changes in wood tissues of sugi (Cryptomeria japonica) resulting from treatment with the ionic liquid 1-ethyl-3-methylimidazolium chloride ([C2mim][Cl]), which dissolves cellulose. Treatment with [C2mim][Cl] caused dissociation and distortion of tracheids in latewood, but not in earlywood. This difference was due to the difference in swelling behavior of the cell wall between earlywood and latewood. Many pit membranes in bordered pits were broken by treatment with [C2mim][Cl]. In addition, some chemical changes in wood components, such as cellulose and lignin, occurred before significant disruption or destruction of the cell wall. Our results show that the reaction of wood liquefaction by [C2mim][Cl] treatment is not homogeneous, both from chemical and morphological viewpoints.
Similar content being viewed by others
Introduction
Energy and environmental issues such as the exhaustion of fossil resources and global warming are major concerns. There is increasing interest in biomass resources as alternatives to fossil resources, because they are renewable and environmentally friendly. Among the various types of biomass, wood is a promising resource, because of its huge stocks and because it is not an edible crop. However, effective conversion technologies are required to use wood for production of bioenergy or bio-based products. There have been many studies on various conversion technologies, including acid hydrolysis, enzymatic saccharification, hot-compressed water treatment, supercritical fluid treatment, pyrolysis, and so on.
Recently, treatment of wood with ionic liquids has been reported as one of the most promising new conversion technologies for biomass. Ionic liquids are organic salts that have melting points close to ambient temperature [1]. These liquids have many notable characteristics, such as negligible vapor pressure, thermal stability, recyclability, and non-flammability. Some ionic liquids can dissolve cellulose [2], and there have been several reports on applications of ionic liquids to liquefy cellulose [3–6] or wood [7–9].
Ionic liquids can liquefy wood more rapidly than the conventional liquefaction system, which consists of phenol with H2SO4 as a catalyst [7]. Wood can be dissolved not only in ionic liquid, but also in a co-solvent mixture of ionic liquid and dimethylsulfoxide (DMSO) [8]. Previously, we reported that wood components such as cellulose, hemicelluloses, and lignin are depolymerized during liquefaction by treatment with an ionic liquid [10]. It was also reported that Japanese beech is more readily liquefied than western red cedar by ionic liquid treatment. These differences in liquefaction behavior were explained by differences in the chemical structure of the lignin between the two wood species [11]. A study of the reaction atmosphere during liquefaction of wood by ionic liquid showed that liquefaction was accelerated in an oxidative atmosphere [12]. Light microscopy studies showed that wood treated with ionic liquid contained cells with swollen and distorted cell walls [13]. However, detailed studies on the morphological changes of wood cell walls have not been carried out yet.
In this study, we conducted detailed analyses of the swelling behavior and distortion of cell walls during the liquefaction of wood by the ionic liquid, 1-ethyl-3-methylimidazolium chloride ([C2mim][Cl]). We used light microscopy and scanning electron microscopy (SEM) to observe and quantify these morphological changes.
Materials and methods
Samples and chemicals
Sugi (Cryptomeria japonica) samples (approx. 5(R) × 5(T) × 20(L) mm) were extracted with ethanol/benzene (1/2, v/v) for 8 h in a Soxhlet apparatus. The extracted wood was oven-dried at 105°C for 24 h prior to use. 1-Ethyl-3-methylimidazolium chloride ([C2mim][Cl]) was purchased from Tokyo Kasei Kogyo Co., Ltd.
Treatment with [C2mim][Cl] for light microscopy analyses
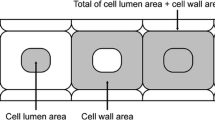
Extracted wood samples were cut with a sliding microtome into 15-μm thick sections, which were mounted in a 20-μm depth hemocytometer (Sunlead Glass Corp.). The mounted section was dried for 2 h at 105°C, then 100 μL of [C2mim][Cl] heated to 120°C was dropped to the mounted section and the hemocytometer was immediately closed with a cover glass; this was designated as the beginning of the treatment. The hemocytometer was placed in an oven at 120°C for various time periods, then removed from the oven to examine the anatomical changes in the wood section by light microscopy (BH-2, OLYMPUS). Three areas (cell lumen area, cell wall area, and total of cell lumen area + cell wall area; defined as shown in Fig. 1) were measured for six neighboring cells in latewood and earlywood using image analysis software (Motic Image Plus 2.25), and the average of each area was calculated.
Treatment with [C2mim][Cl] for scanning electron microscopy (SEM) observations
For SEM observations, extracted wood samples were surfaced with a sliding microtome. The surfaced samples were dried for 24 h at 105°C, and then the surfaced area was treated by dipping into [C2mim][Cl] heated to 120°C for various periods of time. During the dipping treatment, the [C2mim][Cl] was stirred gently with a magnetic stirrer. The treated specimens were dipped into DMSO to remove the [C2mim][Cl], and then washed with distilled water to remove the DMSO. After drying for 24 h at 105°C, the specimen was mounted on a specimen holder and then Pt-coated. The exposed surface was examined by SEM (JEOL JFC-1600) at an accelerating voltage of 25 kV. Any morphological changes of wood cells due to this high accelerating voltage were not observed.
Treatment with [C2mim][Cl] for staining with phloroglucin–HCl
Extracted wood samples were cut into 30-μm thick sections with a sliding microtome, and the sections were mounted on a slide glass. Each mounted section was dried for 2 h at 105°C, and then 15 μL [C2mim][Cl] heated to 120°C was dropped to the surface of the section. The mounted section with [C2mim][Cl] was heated in an oven at 120°C for various periods of time before addition of a few drops of 1% phloroglucin ethanol solution. When the phloroglucin began to form white particles after evaporation of ethanol, lignin in the section was stained by addition of a few drops of 35% hydrochloric acid, and then the section was covered with a coverslip for light microscopy. To evaluate color changes, the colored section was studied with a color difference meter (Nippon Denshoku Kogyo ZE6000) according to the L*a*b* system. The color difference (∆E*) was calculated using the following formula:
where ∆L* is the difference in brightness of the colored section before and after [C2mim][Cl] treatment, and ∆a* and ∆b* are differences in chroma of the colored section before and after [C2mim][Cl] treatment.
Results and discussion
Using light microscopy, we examined wood samples at 0 and 24 h of treatment with [C2mim][Cl] at 120°C (Fig. 2). In the transverse sections, we observed that cell walls in latewood were well ordered at 0 h (Fig. 2a), but disordered and distorted after 24 h of treatment (Fig. 2b). In contrast, no significant morphological changes were observed in earlywood. Although the cell walls in earlywood swelled as a result of [C2mim][Cl] treatment, the cells retained a similar form to that before treatment. In the radial section, we observed dissociation of tracheids at the boundary regions of latewood and earlywood after 24 h of treatment (Fig. 2d). Moreover, after 24 h of treatment, the tracheids were distorted in latewood but not in earlywood. Distorted tracheids were also visible in the tangential sections of wood samples after 24 h of treatment (Fig. 2f).
To analyze in detail the dissociations and distortions in latewood resulting from [C2mim][Cl] treatment, we used image analysis software to analyze the swelling behavior of cell walls in latewood and earlywood in transverse sections. Swelling of cell walls was evaluated by determining the cell lumen area, cell wall area, and total of cell lumen area + cell wall area (as defined in Fig. 1); the results are shown in Fig. 3. In earlywood (upper panel, Fig. 3), the cell lumen area decreased with increasing cell wall area at an early stage of [C2mim][Cl] treatment. After these initial changes in cell lumen area and cell wall area, both areas remained stable thereafter and did not show further changes during the [C2mim][Cl] treatment. Meanwhile, the total of cell lumen area + cell wall area was found to be almost constant with keeping initial area during the [C2mim][Cl] treatment. These results indicate that cell walls of tracheids in earlywood swelled toward the cell lumen without swelling outwards, but did not break or dissociate (Fig. 2). In latewood (lower panel, Fig. 3), the cell lumen area decreased to nearly zero, and there were marked increases in the cell wall area and total of cell lumen area + cell wall area at an early stage of [C2mim][Cl] treatment. After these changes, all three areas increased gradually with increasing treatment time. At 48 h of treatment, the cell wall area and total of cell lumen area + cell wall area had increased by 5 and 2.5 times, respectively. These results indicate that tracheids in latewood swelled toward the cell lumen at an early stage of [C2mim][Cl] treatment. In latewood, the cell lumen was soon occluded by the swelling of the cell wall, because the area of the lumen is much smaller in latewood than in earlywood. In addition, the tracheids in latewood swelled outwards at an early stage of [C2mim][Cl] treatment, unlike in earlywood. This swelling outwards likely caused the dissociation and distortions in latewood. Once the tracheids have dissociated, their cell walls can swell freely because there are no longer the physical restraints of neighboring cell walls. Thus, the cell walls continued to swell gradually with increases in the total of cell lumen + cell wall area. These results indicate that the differences in the morphological changes between earlywood and latewood (Fig. 2) were because of differences in swelling behavior (as shown in Fig. 3).
We analyzed transverse sections of wood samples after a 24-h [C2mim][Cl] treatment by SEM. We observed dissociated and distorted cell walls in latewood after 24 h of treatment (Fig. 4b), as observed by light microscopy (Fig. 2). The magnified SEM image in Fig. 4d shows the dissociation between the secondary cell wall and the intercellular layer (indicated by arrows). This dissociation is not due to the pretreatment for SEM observation such as drying, Pt-coating and so on, because it can be observed by light microscopy as in our previous study [13]. The reasons for this dissociation are unclear; however, it may be related to differences in the composition of the two layers. The secondary cell wall contains 44% cellulose, 34% hemicelluloses, and 22% lignin, whereas the intercellular layer contains 9% cellulose, 20% hemicelluloses, and 71% lignin [14]. Cellulose and hemicelluloses are liquefied more readily than lignin by [C2mim][Cl] treatment [10, 11]. Therefore, the reaction behaviors of the secondary cell wall and the intercellular layer are expected to differ because of their different compositions. We can speculate that these differences in reaction behavior also result in differences in their swelling behavior, and consequently, the dissociation between the secondary cell wall and intercellular layer.
We analyzed radial sections of wood samples after a 24-h [C2mim][Cl] treatment using SEM (Fig. 5). In latewood, the tracheids dissociated and showed flaking and distortion (Fig. 5b), and ray tracheids became segmented (indicated by arrows in Fig. 5d). These results indicated that the swelling of the tracheids was greater in the radial direction than in the axial direction. SEM analyses of tangential sections of wood samples showed that the tracheids present in latewood before treatment (0 h, Fig. 6a) disappeared after the 24-h [C2mim][Cl] treatment (Fig. 6b). As shown in Figs. 2, 4, and 5, the latewood was degraded as a result of significant morphological changes, i.e., dissociations and distortions of cell walls.
Figure 7 shows SEM images of a bordered pit in earlywood in a radial section. Many pit membranes in bordered pits were broken after 48 h of treatment (Fig. 7b, d), although all pits were occluded by tori before treatment (Fig. 7a, c). Figure 8 shows SEM images of a bordered pit-pair in a tangential section of earlywood. Before the treatment, the torus is present in the bordered pit-pair (arrow, 0 h, Fig. 8a, c); however, it disappears after 48 h of treatment (Fig. 8b, d) even though the bordered pit-pair remains intact and unchanged. As shown in Figs. 4, 5, and 6, the tracheids in earlywood did not show significant morphological changes. The pit membrane is constructed mainly from cellulose microfibrils [15]. In our previous paper on liquefaction of wood, we reported that cellulose is liquefied more readily than hemicelluloses and lignin [10, 11]. Because the pit membranes consist of cellulose, they were largely destroyed by [C2mim][Cl] treatment as shown in Figs. 7 and 8.
Figure 9 shows light micrographs and polarizing microscopy images of a transverse section treated with [C2mim][Cl] at 120°C for various periods of time. The cell walls in latewood became disordered and distorted, as shown in Fig. 2. The brightness due to the birefringence of cellulose is visible in intact samples in the polarization image at 0 h treatment (Fig. 9a). The brightness decreases gradually with increasing treatment time (Fig. 9b, c). These results indicate that the crystalline structures of cellulose in wood are destroyed before the wood cells liquefy completely during [C2mim][Cl] treatment.
Figure 10 shows light micrographs of three sections stained via the phloroglucin reaction after treatment by [C2mim][Cl] at 120°C for 72 h. In the intact cells (0 h treatment), the cells show a red color because of the reaction of phloroglucin with the aldehyde groups of coniferylaldehyde structures in lignin (Fig. 10, upper panels). However, after 72 h of treatment, the red color is diminished (Fig. 10, lower panels). To quantify the color changes, we determined the color difference (∆E*) with a color difference meter (Fig. 11). In all sections, ∆E* increased with increasing treatment time, indicating that the chemical structure in lignin was altered by [C2mim][Cl] treatment. The results shown in Figs. 9, 10, and 11 demonstrate that during [C2mim][Cl] treatment, some chemical changes in wood components such as cellulose and lignin occur before the cell wall is ruptured or destroyed.
Conclusion
Wood can be liquefied by treatment with [C2mim][Cl] [10, 11]. In our previous studies on liquefaction behavior of wood by treatment with [C2mim][Cl] [10, 11], we reported that polysaccharides such as cellulose and hemicelluloses were liquefied more readily than lignin, indicating that the chemical reaction of wood in [C2mim][Cl] is not homogeneous. In the present study, our results showed that treatment with [C2mim][Cl] resulted in distortion and dissociation of cell walls in latewood, but not in earlywood. In addition, [C2mim][Cl] treatment resulted in chemical changes in wood components such as cellulose and lignin without significant collapse of the cell wall. These results indicate that the morphological changes in cells during [C2mim][Cl] treatment are not homogeneous. Consequently, we can conclude that the liquefaction of wood by [C2mim][Cl] is not homogeneous, both from chemical and morphological viewpoints.
References
Seddon KR (1997) Ionic liquids for clean technology. J Chem Technol Biotechnol 68:351–356
Swatloski RP, Spear SK, Hobrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4947–4975
Turner MB, Spear SK, Holbrey JD, Rogers RD (2004) Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 5:1379–1384
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–307
Xie H, Shi T (2006) Wood liquefaction by ionic liquids. Holzforschung 60:509–512
Fort DA, Remsing RC, Swatloski RP, Moyna P, Moyna P, Rogers RD (2007) Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem 9:63–69
Kilpeläinen I, Xie H, King A, Granstrom M, Heikkinen S, Argyropoulos DS (2007) Dissolution of wood in ionic liquids. J Agric Food Chem 55:9142–9148
Miyafuji H, Miyata K, Saka S, Ueda F, Mori M (2009) Reaction behavior of wood in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 55:215–219
Nakamura A, Miyafuji H, Saka S (2010) Liquefaction behaviour of Western red cedar and Japanese beech in the ionic liquid 1-ethyl-3-methylimidazolium chloride. Holzforschung 64:289–294
Nakamura A, Miyafuji H, Saka S (2010) Influence of reaction atmosphere on the liquefaction and depolymerisation of wood in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 56:256–261
Miyafuji H, Suzuki N (2011) Observation by light microscope of sugi (Cryptomeria japonica) treated with the ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 57:459–461
Harada H (1985) Cell wall (in Japanese). In: Harada H, Saiki H (eds) Structure of wood. Buneido Shuppan, Tokyo, pp 148–151
Tsoumis G (1991) Chemical composition and ultrastructure of wood. In: Tsoumis G (ed) Science and technology of Wood. Van Nostrand Reinhold, New York, pp 42–46
Acknowledgments
This research was supported in part by the TOSTEM Foundation for Construction Materials Industry Promotion.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Miyafuji, H., Suzuki, N. Morphological changes in sugi (Cryptomeria japonica) wood after treatment with the ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 58, 222–230 (2012). https://doi.org/10.1007/s10086-011-1245-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-011-1245-3