Abstract
Background
Stroke is a significant global cause of mortality and morbidity, and post-stroke cognitive impairment (PSCI) affects up to half of stroke patients. Despite the availability of pharmacological and non-pharmacological interventions, there is a lack of definitive effective treatments for PSCI. Non-invasive brain stimulation, particularly intermittent theta burst stimulation (iTBS), has emerged as a promising therapy for the treatment of PSCI.
Objective
This systematic review and meta-analysis aimed to evaluate the efficacy and safety of iTBS in enhancing cognitive function among patients with PSCI.
Methods
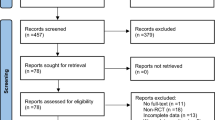
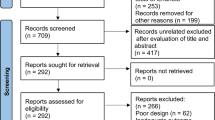
A comprehensive search was conducted across multiple databases, including PubMed, Web of Science, Scopus, Cochrane Library, and CNKI, to identify relevant randomized controlled trials published before April 2023. The primary outcome measured changes in global cognitive scales, while the secondary outcomes focused on improvements in attention, orientation, visual-spatial perception, and activities of daily living.
Results
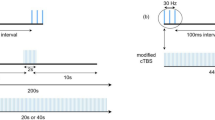
The meta-analysis encompassed six studies involving 325 patients. The results demonstrated that iTBS led to a significant improvement in global cognitive scales (SMD = 1.12, 95% CI = [0.59 to 1.65], P < 0.0001), attention (SMD = 0.48, 95% CI [0.13 to 0.82], P = 0.007), visual perception (SMD = 0.99, 95% CI [0.13 to 1.86], P = 0.02), and activities of daily living (SMD = 0.82, 95% CI [0.55 to 1.08], P < 0.00001). However, there was no significant effect on orientation (SMD = 0.36, 95% CI [− 0.04 to 0.76], P = 0.07). Subgroup analysis based on the number of sessions was conducted, revealing a significant improvement in global cognition among patients with PSCI across the three categories (10 sessions, 20 sessions, and 30 sessions) with no between-group difference (P = 0.28). None of the included studies reported any serious adverse effects.
Conclusion
In conclusion, iTBS appears to be a safe and effective non-invasive treatment that can enhance the cognitive abilities and daily living skills of patients with post-stroke cognitive impairment. However, our conclusion is constrained by the limited number of studies. Further high-quality, large-sample RCTs with extended follow-up periods are necessary to validate these findings. Integrating iTBS with brain imaging techniques, such as functional near-infrared spectroscopy and functional magnetic resonance, could aid in understanding the mechanism of iTBS action.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Guarantor: Moaz Elsayed.
References
Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P (2022) World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke 17(1):18–29. https://doi.org/10.1177/17474930211065917
Shen W, Fan X, Wang L, Zhang Y (2022) Traditional Chinese medicine for post-stroke cognitive impairment: a systematic review and meta-analysis. Front Pharmacol 13. https://doi.org/10.3389/fphar.2022.816333
Sun J-H, Tan L, Yu J-T (2014) Post-stroke cognitive impairment: epidemiology, mechanisms, and management. Ann Transl Med 2(8):80. https://doi.org/10.3978/j.issn.2305-5839.2014.08.05
Lanza G, Bramanti P, Cantone M, Pennisi M, Pennisi G, Bella R (2017) Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav Neurol 2017:1–16. https://doi.org/10.1155/2017/1421326
Li K-P, Sun J, Wu C-Q, An X-F, Wu J-J, Zheng M-X, Hua X-Y, Xu J-G (2023) Effects of repetitive transcranial magnetic stimulation on post-stroke patients with cognitive impairment: a systematic review and meta-analysis. Behav Brain Res 439:114229. https://doi.org/10.1016/j.bbr.2022.114229
Solomon EA, Sperling MR, Sharan AD, Wanda PA, Levy DF, Lyalenko A, Pedisich I, Rizzuto DS, Kahana MJ (2021) Theta-burst stimulation entrains frequency-specific oscillatory responses. Brain Stimul 14(5):1271–1284. https://doi.org/10.1016/j.brs.2021.08.014
Herrero JL, Smith A, Mishra A, Markowitz N, Mehta AD, Bickel S (2021) Inducing neuroplasticity through intracranial θ-burst stimulation in the human sensorimotor cortex. J Neurophysiol 126(5):1723–1739. https://doi.org/10.1152/jn.00320.2021
Mendlowitz AB, Shanbour A, Downar J, Vila-Rodriguez F, Daskalakis ZJ, Isaranuwatchai W, Blumberger DM (2019) Implementation of intermittent theta burst stimulation compared to conventional repetitive transcranial magnetic stimulation in patients with treatment-resistant depression: a cost analysis. PLoS ONE 14(9):e0222546–e0222546. https://doi.org/10.1371/journal.pone.0222546
Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC (2016) Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul 9(3):323–335. https://doi.org/10.1016/j.brs.2016.01.006
Gamboa OL, Antal A, Moliadze V, Paulus W (2010) Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res 204(2):181–187. https://doi.org/10.1007/s00221-010-2293-4
Hoy KE, Bailey N, Michael M, Fitzgibbon B, Rogasch NC, Saeki T, Fitzgerald PB (2015) Enhancement of working memory and task-related oscillatory activity following intermittent theta burst stimulation in healthy controls. Cereb Cortex 26(12):4563–4573. https://doi.org/10.1093/cercor/bhv193
Trung J, Hanganu A, Jobert S, Degroot C, Mejia-Constain B, Kibreab M, Bruneau M-A, Lafontaine A-L, Strafella A, Monchi O (2019) Transcranial magnetic stimulation improves cognition over time in Parkinson’s disease. Parkinsonism Relat Disord 66:3–8
Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M (2012) Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm 120(5):813–819. https://doi.org/10.1007/s00702-012-0902-z
Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH (2022) Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. Available from. http://www.training.cochrane.org/handbook
Higgins J, Deeks J, Altman D (2011) Special topics in statistics. In J. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions (Version 5.1.0). The Cochrane Collaboration. Retrieved April 30, 2023, from. http://handbook.cochrane.org
WebPlotDigitizer 4.6. https://apps.automeris.io/wpd/
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range, and/or interquartile range. BMC Med Res Methodol 14(1). https://doi.org/10.1186/1471-2288-14-135
Luo D, Wan X, Liu J, Tong T (2016) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27(6):1785–1805. https://doi.org/10.1177/0962280216669183
Tsai PY, Lin WS, Tsai KT, Kuo CY, Lin PH (2020) High frequency versus theta burst transcranial magnetic stimulation for the treatment of poststroke cognitive impairment in humans. J Psychiatr Neurosci: JPN 45(4):262–270. https://doi.org/10.1503/jpn.190060
Li W, Wen Q, Xie YH, Hu AL, Wu Q, Wang YX (2022) Improvement of poststroke cognitive impairment by intermittent theta bursts: a double-blind randomized controlled trial. Brain Behav 12(6):e2569. https://doi.org/10.1002/brb3.2569
Chu M, Zhang Y, Chen J, Chen W, Hong Z, Zhang Y, Yu H, Zhang F, Ye X, Li J, Yang Y (2022) Efficacy of intermittent theta-burst stimulation and transcranial direct current stimulation in treatment of post-stroke cognitive impairment. J Integr Neurosci 21(5):130. https://doi.org/10.31083/j.jin2105130
Wenbin Yi, Qixiu Z, Naisu T et al (2020) Effects of intermittent theta short burst rapid pulse transcranial magnetic stimulation on depression and cognitive function in stroke patients [J]. J Precis Med 35(04):347–350. https://doi.org/10.13362/j.jpmed.202004016
Yanfang G, Yongrui L, Weili W et al (2023) Effect of intermittent theta rhythm stimulation on patients with post-cerebral infarction depression and cognitive impairment [J]. Int J Psychiatr 50(01):121–124. https://doi.org/10.13479/j.cnki.jip.2023.01.045
Song P, Jian W (2022) Effect of repetitive transcranial magnetic intermittent theta burst stimulation on post-stroke cognitive impairment. Chongqing Medicine 裴松,王健 & 夏家怡. 重复经颅磁间歇性θ节律刺激对卒中后认知功能障碍的疗效观察 51(18):3120–3125
Bour A, Rasquin S, Boreas A, Limburg M, Verhey F (2010) How predictive is the MMSE for cognitive performance after stroke? J Neurol 257(4):630–637. https://doi.org/10.1007/s00415-009-5387-9
Schmitt AL, Livingston RB, Goette WF, Galusha-Glasscock JM (2016) Relationship between the mini-mental state examination and the repeatable battery for the assessment of neuropsychological status in patients referred for a dementia evaluation. Percept Mot Skills 123(3):606–623. https://doi.org/10.1177/0031512516667674
Wang SY, Gong ZK, Sen J, Han L, Zhang M, Chen W (2014) The usefulness of the Loewenstein Occupational Therapy Cognition Assessment in evaluating cognitive function in patients with stroke. Eur Rev Med Pharmacol Sci 18(23):3665–3672
Singh A, Richardson SP, Narayanan N, Cavanagh JF (2018) Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia 117:113–122. https://doi.org/10.1016/j.neuropsychologia.2018.05.020
Trujillo P, van Wouwe NC, Lin Y-C, Stark AJ, Petersen KJ, Kang H et al (2019) Dopamine effects on frontal cortical blood flow and motor inhibition in Parkinson’s disease. Cortex 115:99–111. https://doi.org/10.1016/j.cortex.2019.01.016
Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Öngür D (2014) Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiat 71(2):109–118. https://doi.org/10.1001/jamapsychiatry.2013.3469
He W, Wang JC, Tsai PY (2021) Theta burst magnetic stimulation improves Parkinson’s-related cognitive impairment: a randomised controlled study. Neurorehabil Neural Repair 35(11):986–995. https://doi.org/10.1177/1545968321104131
Liang P, Wang Z, Yang Y, Jia X, Li K (2011) Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS ONE 6:e22153
Kim J, Cha B, Lee D, Kim JM, Kim M (2022) Effect of cognition recovery by repetitive transcranial magnetic stimulation on ipsilesional dorsolateral prefrontal cortex in subacute stroke patients. Front Neurol 13:823108. https://doi.org/10.3389/fneur.2022.823108
Wozniak-Kwasniewska A, Szekely D, Aussedat P et al (2014) Changes of oscillatory brain activity induced by repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in healthy subjects. Neuroimage 88:91–99
Koch G, Di Lorenzo F, Bonnì S, Giacobbe V, Bozzali M, Caltagirone C, Martorana A (2014) Dopaminergic modulation of cortical plasticity in Alzheimer’s disease patients. Neuropsychopharmacology 39(11):2654–2661
Li KP, Sun J, Wu CQ et al (2023) Effects of repetitive transcranial magnetic stimulation on post-stroke patients with cognitive impairment: a systematic review and meta-analysis. Behav Brain Res 439:114229. https://doi.org/10.1016/j.bbr.2022.114229
Wang Y, Xu N, Wang R, Zai W (2022) Systematic review and network meta-analysis of effects of noninvasive brain stimulation on post-stroke cognitive impairment. Front Neurosci. 16:1082383. https://doi.org/10.3389/fnins.2022.1082383. (Published 2022 Dec 28)
Acknowledgements
The authors would like to thank Dr. Rehab Adel Diab and Dr. Ahmed Negida for providing support and advice as well as helping with the revision of this paper.
Author information
Authors and Affiliations
Contributions
• Study conceptualization and design: Asmaa Daoud, Moaz Elsayed.
• Protocol design: all authors
• Abstract screening on Rayyan, full-text screening and study selection, data extraction, and quality assessment: all authors
• Data analysis: Asma Daoud, Moaz Elsayed
• Writing: all authors contributed in the following order: Moaz Elsayed, Asmaa Daoud, Asmaa Zakria Alnajjar, Abdulrahman Krayim, Maickel AbdelMeseh, Taleb Alsalloum, Yehia Nabil, Roaa Faisal
• Figures and tables: Asmaa Zakria Alnajjar, Abdulrahman Krayim
• Proofreading and revision: Moaz Elsayed, Rehab Diab, Ahmed Negida
Corresponding author
Ethics declarations
Informed consent and ethical approval
Informed consent and Ethical approval were not sought for this paper because it included no direct interaction with human participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Register number PROSPERO 2023: CRD42023413507.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daoud, A., Elsayed, M., Alnajjar, A.Z. et al. Efficacy of intermittent theta burst stimulation (iTBS) on post-stroke cognitive impairment (PSCI): a systematic review and meta-analysis. Neurol Sci 45, 2107–2118 (2024). https://doi.org/10.1007/s10072-023-07267-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07267-w