Abstract
Aims
To analyze the clinical characteristics of acute symptomatic seizures and predict the risk factors for seizure recurrence in patients with anti-N-methyl-d-aspartate receptor (NMDAR), anti-leucine-rich glioma-inactivated 1 (LGI1), and anti-gamma-aminobutyric acid B receptor (GABABR) encephalitis.
Methods
In this retrospective study, we included hospitalized patients who had been diagnosed with anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis between November 2014 and April 2021. Binary logistic regression analysis was performed to identify the potential risk factors for seizure recurrence.
Results
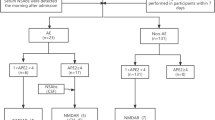
In total, 262 patients with anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis were included, 197 (75.2%) of whom presented with acute symptomatic seizures. During follow-up, 42 patients exhibited seizure recurrence. In anti-NMDAR encephalitis, frontal lobe abnormality on brain magnetic resonance imaging, delayed immunotherapy, early seizures, and focal motor onset were associated with seizure recurrence.
Conclusions
Acute symptomatic seizure is a common clinical feature observed in patients with anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis, with 50% of patients presenting with seizures as an initial symptom. The prognosis of patients with acute symptomatic seizures can be improved after receiving immunotherapy. Nevertheless, a minority of patients will experience seizure recurrence; therefore, restarting immunotherapy is recommended.



Similar content being viewed by others
Data availability
The raw data supporting the findings of our study is available from the corresponding author upon reasonable request.
References
Dalmau J, Geis C, Graus F (2017) Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev 97(2):839–887
Dalmau J, Ropper AH, Graus F (2018) Antibody-mediated encephalitis. N Engl J Med 378(9):840–851
Husari KS, Dubey D (2019) Autoimmune epilepsy. Neurotherapeutics 16(3):685–702
Kaaden T et al (2022) Seizure semiology in antibody-associated autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 9(6)
Liu X et al (2022) Long-term seizure outcomes in patients with autoimmune encephalitis: a prospective observational registry study update. Epilepsia 63(7):1812–1821
Zhong R et al (2022) Acute symptomatic seizures and risk of epilepsy in autoimmune encephalitis: a retrospective cohort study. Front Immunol 13
de Bruijn M et al (2019) Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology 92(19):e2185–e2196
Chen SS et al (2021) Predictors and prognoses of epilepsy after anti-neuronal antibody-positive autoimmune encephalitis. Seizure 92:189–194
Lin J et al (2019) Encephalitis with antibodies against the GABA(B) receptor: high mortality and risk factors. Front Neurol 10:1030
Steriade C et al (2020) Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia 61(7):1341–1351
Thompson J et al (2018) The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141(2):348–356
Ilyas-Feldmann M, Pruss H, Holtkamp M (2021) Long-term seizure outcome and antiseizure medication use in autoimmune encephalitis. Seizure 86:138–143
Yao L et al (2019) Clinical features and long-term outcomes of seizures associated with autoimmune encephalitis: a follow-up study in East China. J Clin Neurosci 68:73–79
Gifreu A et al (2021) Risk of developing epilepsy after autoimmune encephalitis. Brain Sci 11(9)
Graus F et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Fisher RS et al (2017) Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58(4):522–530
van Sonderen A et al (2016) Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87(14):1449–1456
Trinka E et al (2015) A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 56(10):1515–1523
Balu R et al (2019) A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 92(3):e244–e252
Spatola M, Dalmau J (2017) Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol 30(3):345–353
Gillinder L, Britton J (2022) Autoimmune-associated seizures. Continuum (Minneap Minn) 28(2):363–398
Shen CH et al (2020) Seizures and risk of epilepsy in anti-NMDAR, anti-LGI1, and anti-GABAB R encephalitis. Ann Clin Transl Neurol 7(8):1392–1399
Yeshokumar AK et al (2021) Seizures in autoimmune encephalitis-a systematic review and quantitative synthesis. Epilepsia 62(2):397–407
Wang Y et al (2021) Characteristics and outcome-related factors of seizure at the first onset of autoimmune encephalitis: a retrospective study. CNS Neurosci Ther 27(6):694–701
Irani SR et al (2013) Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 136(Pt 10):3151–3162
Lin N et al (2021) Long-term seizure outcomes in patients with anti-Leucine-rich glioma-inactivated 1 encephalitis. Epilepsy Behav 122:108159
Singh TD et al (2015) Postencephalitic epilepsy: clinical characteristics and predictors. Epilepsia 56(1):133–138
Bladin CF et al (2000) Seizures after stroke - a prospective multicenter study. Arch Neurol 57(11):1617–1622
Huang Q et al (2018) Characteristics of seizure and antiepileptic drug utilization in outpatients with autoimmune encephalitis. Front Neurol 9:1136
Carreño M et al (2017) Epilepsy surgery in drug resistant temporal lobe epilepsy associated with neuronal antibodies. Epilepsy Res 129:101–105
Acknowledgements
We wish to express our gratitude to all the individuals who participated in this study.
Funding
This study received support from the National Natural Science Foundation of China (No.: 81771397).
Author information
Authors and Affiliations
Contributions
DG Cui collected the data and drafted the main manuscript. JL Feng, M Yang, and YY Dong collected the data. YJ Lian revised the manuscript and designed the study.
Corresponding author
Ethics declarations
Consent to participate
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Number:2021-KY-0779-002). Written informed consent to participate was obtained from the patient or his/her legal guardian. This study will not disclose personal information of patients.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, D., Feng, J., Yang, M. et al. Acute Symptomatic Seizures and Risk of Seizure Recurrence in Patients with Anti-NMDAR, Anti-LGI1, and Anti-GABABR Encephalitis. Neurol Sci 45, 1609–1617 (2024). https://doi.org/10.1007/s10072-023-07165-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07165-1