Abstract
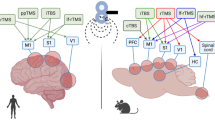
Repetitive transcranial magnetic stimulation (rTMS) has been widely used in motor rehabilitation after stroke, and functional magnetic resonance imaging (fMRI) has been used to investigate the neural mechanisms of motor recovery during stroke therapy. However, there is no review on the mechanism of rTMS intervention for motor recovery after stroke based on fMRI explicitly. We aim to reveal and summarize the neural mechanism of the effects of rTMS on motor function after stroke as measured by fMRI. We carefully performed a literature search using PubMed, EMBASE, Web of Science, and Cochrane Library databases from their respective inceptions to November 2022 to identify any relevant randomized controlled trials. Researchers independently screened the literature, extracted data, and qualitatively described the included studies. Eleven studies with a total of 420 poststroke patients were finally included in this systematic review. A total of 338 of those participants received fMRI examinations before and after rTMS intervention. Five studies reported the effects of rTMS on activation of brain regions, and four studies reported results related to brain functional connectivity (FC). Additionally, five studies analyzed the correlation between fMRI and motor evaluation. The neural mechanism of rTMS in improving motor function after stroke may be the activation and FCs of motor-related brain areas, including enhancement of the activation of motor-related brain areas in the affected hemisphere, inhibition of the activation of motor-related brain areas in the unaffected hemisphere, and changing the FCs of intra-hemispheric and inter-hemispheric motor networks.



Similar content being viewed by others
Data availability
Data sharing is not applicable as no datasets were generated or analyzed in this study.
References
Kuriakose D, Xiao Z (2020) Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci 21(20). https://doi.org/10.3390/ijms21207609
Peisker T, Koznar B, Stetkarova I, Widimsky P (2017) Acute stroke therapy: a review. Trends Cardiovasc Med 27(1):59–66. https://doi.org/10.1016/j.tcm.2016.06.009
Gou X, Xu D, Li F, Hou K, Fang W, Li Y (2021) Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J Physiol Biochem 77(4):511–529. https://doi.org/10.1007/s13105-021-00817-w
Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):439–458. https://doi.org/10.1016/S1474-4422(19)30034-1
Langhorne P, Coupar F, Pollock A (2009) Motor recovery after stroke: a systematic review. Lancet Neurol 8(8):741–754. https://doi.org/10.1016/S1474-4422(09)70150-4
Stinear CM (2017) Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 16(10):826–836. https://doi.org/10.1016/S1474-4422(17)30283-1
Somaa FA, de Graaf TA, Sack AT (2022) Transcranial magnetic stimulation in the treatment of neurological diseases. Front Neurol 13:793253. https://doi.org/10.3389/fneur.2022.793253
Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M (2019) Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord 12:1278099885. https://doi.org/10.1177/1756286419878317
Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 131(2):474–528. https://doi.org/10.1016/j.clinph.2019.11.002
Schambra HM (2018) Repetitive transcranial magnetic stimulation for upper extremity motor recovery: does it help? Curr Neurol Neurosci Rep 18(12):97. https://doi.org/10.1007/s11910-018-0913-8
Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2(3):145–156. https://doi.org/10.1016/s1474-4422(03)00321-1
Gilio F, Conte A, Vanacore N, Frasca V, Inghilleri M, Berardelli A (2007) Excitatory and inhibitory after-effects after repetitive magnetic transcranial stimulation (rTMS) in normal subjects. Exp Brain Res 176(4):588–593. https://doi.org/10.1007/s00221-006-0638-9
Tang Z, Han K, Wang R, Zhang Y, Zhang H (2022) Excitatory repetitive transcranial magnetic stimulation over the ipsilesional hemisphere for upper limb motor function after stroke: a systematic review and meta-analysis. Front Neurol 13:918597. https://doi.org/10.3389/fneur.2022.918597
Bai Z, Zhang J, Fong K (2022) Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 19(1):24. https://doi.org/10.1186/s12984-022-00999-4
Gutiérrez-Muto AM, Castilla J, Freire M, Oliviero A, Tornero J (2020) Theta burst stimulation: technical aspects about TMS devices. Brain Stimul 13(3):562–564. https://doi.org/10.1016/j.brs.2020.01.002
Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC (2016) Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul 9(3):323–335. https://doi.org/10.1016/j.brs.2016.01.006
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005) Theta burst stimulation of the human motor cortex. Neuron 45(2):201–206. https://doi.org/10.1016/j.neuron.2004.12.033
Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V (2014) Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 10(10):597–608. https://doi.org/10.1038/nrneurol.2014.162
Simonetta-Moreau M (2014) Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann Phys Rehabil Med 57(8):530–542. https://doi.org/10.1016/j.rehab.2014.08.003
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123(Pt 3):572–584. https://doi.org/10.1093/brain/123.3.572
Guidali G, Roncoroni C, Bolognini N (2021) Modulating frontal networks’ timing-dependent-like plasticity with paired associative stimulation protocols: recent advances and future perspectives. Front Hum Neurosci 15:658723. https://doi.org/10.3389/fnhum.2021.658723
Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J (2002) Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543(Pt 2):699–708. https://doi.org/10.1113/jphysiol.2002.023317
Hu Y, Guo TC, Zhang XY, Tian J, Lu YS (2019) Paired associative stimulation improves synaptic plasticity and functional outcomes after cerebral ischemia. Neural Regen Res 14(11):1968–1976. https://doi.org/10.4103/1673-5374.259618
Carson RG, Kennedy NC (2013) Modulation of human corticospinal excitability by paired associative stimulation. Front Hum Neurosci 7:823. https://doi.org/10.3389/fnhum.2013.00823
Du J, Tian L, Liu W, Hu J, Xu G, Ma M, Fan X, Ye R, Jiang Y, Yin Q, Zhu W, Xiong Y, Yang F, Liu X (2016) Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol 23(11):1666–1672. https://doi.org/10.1111/ene.13105
Moslemi HF, Kordi YA, Razeghi M, Shariat A, Bagheri Z, Rezaei K (2021) The effect of high-frequency repetitive transcranial magnetic stimulation on functional indices of affected upper limb in patients with subacute stroke. J Biomed Phys Eng 11(2):175–184. https://doi.org/10.31661/jbpe.v0i0.879
Luk KY, Ouyang HX, Pang M (2022) Low-frequency rTMS over contralesional M1 increases ipsilesional cortical excitability and motor function with decreased interhemispheric asymmetry in subacute stroke: a randomized controlled study. Neural Plast 2022:3815357. https://doi.org/10.1155/2022/3815357
Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG (2012) Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology 78(4):256–264. https://doi.org/10.1212/WNL.0b013e3182436558
Guo Z, Jin Y, Bai X, Jiang B, He L, McClure MA, Mu Q (2021) Distinction of high- and low-frequency repetitive transcranial magnetic stimulation on the functional reorganization of the motor network in stroke patients. Neural Plast 2021:8873221. https://doi.org/10.1155/2021/8873221
Zhang JJ, Bai Z, Fong K (2022) Priming intermittent theta burst stimulation for hemiparetic upper limb after stroke: a randomized controlled trial. Stroke 53(7):2171–2181. https://doi.org/10.1161/STROKEAHA.121.037870
Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH (2011) Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 42(5):1357–1362. https://doi.org/10.1161/STROKEAHA.110.596155
Golestani AM, Tymchuk S, Demchuk A, Goodyear BG (2013) Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair 27(2):153–163. https://doi.org/10.1177/1545968312457827
Lam TK, Dawson DR, Honjo K, Ross B, Binns MA, Stuss DT, Black SE, Chen JJ, Levine BT, Fujioka T, Chen JL (2018) Neural coupling between contralesional motor and frontoparietal networks correlates with motor ability in individuals with chronic stroke. J Neurol Sci 384:21–29. https://doi.org/10.1016/j.jns.2017.11.007
Peng Y, Liu J, Hua M, Liang M, Yu C (2019) Enhanced effective connectivity from ipsilesional to contralesional M1 in well-recovered subcortical stroke patients. Front Neurol 10:909. https://doi.org/10.3389/fneur.2019.00909
Frahm J, Bruhn H, Merboldt KD, Hänicke W (1992) Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging 2(5):501–505. https://doi.org/10.1002/jmri.1880020505
Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Et A (1992) Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89(12):5675–5679. https://doi.org/10.1073/pnas.89.12.5675
Turner R, Howseman A, Rees GE, Josephs O, Friston K (1998) Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res 123(1–2):5–12. https://doi.org/10.1007/s002210050538
Li B, Deng S, Sang B, Zhu W, Zhuo B, Zhang M, Qin C, Lyu Y, Du Y, Meng Z (2022) Revealing the neuroimaging mechanism of acupuncture for poststroke aphasia: a systematic review. Neural Plast 2022:5635596. https://doi.org/10.1155/2022/5635596
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711. https://doi.org/10.1038/nrn2201
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83(8):713–721
de Morton NA (2009) The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 55(2):129–133. https://doi.org/10.1016/s0004-9514(09)70043-1
Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, Fink GR, Nowak DA (2009) Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66(3):298–309. https://doi.org/10.1002/ana.21725
Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, Pascual-Leone A (2012) rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci 30(3):179–189. https://doi.org/10.3233/RNN-2012-110162
Chen Q, Shen W, Sun H, Zhang H, Liu C, Chen Z, Yu L, Cai X, Ke J, Li L, Zhang L, Fang Q (2022) The effect of coupled inhibitory-facilitatory repetitive transcranial magnetic stimulation on shaping early reorganization of the motor network after stroke. Brain Res 1790:147959. https://doi.org/10.1016/j.brainres.2022.147959
Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, Zhang Q, Liu L, Mantini D, Zhang Z, Lu G, Liu X (2019) Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin 21:101620. https://doi.org/10.1016/j.nicl.2018.101620
Juan D, Yao W, Li J, Yang F, Hu J, Xu Q, Liu L, Lv Q, Liu R, Ye R, Ma M, Zhu W, Zhang Z, Liu X (2022) Motor network reorganization after repetitive transcranial magnetic stimulation in early stroke patients: a resting state fMRI study. Neurorehabil Neural Repair 36(1):61–68. https://doi.org/10.1177/15459683211054184
Gottlieb A, Boltzmann M, Schmidt SB, Gutenbrunner C, Krauss JK, Stangel M, Höglinger GU, Wallesch CW, Rollnik JD (2021) Treatment of upper limb spasticity with inhibitory repetitive transcranial magnetic stimulation: a randomized placebo-controlled trial. NeuroRehabilitation 49(3):425–434. https://doi.org/10.3233/NRE-210088
Li J, Zhang XW, Zuo ZT, Lu J, Meng CL, Fang HY, Xue R, Fan Y, Guan YZ, Zhang WH (2016) Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fMRI study. Cns Neurosci Ther 22(12):952–960. https://doi.org/10.1111/cns.12593
Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Küst J, Karbe H, Fink GR (2008) Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol 65(6):741–747. https://doi.org/10.1001/archneur.65.6.741
Qin Y, Liu X, Guo X, Liu M, Li H, Xu S (2021) Low-frequency repetitive transcranial magnetic stimulation restores dynamic functional connectivity in subcortical stroke. Front Neurol 12:771034. https://doi.org/10.3389/fneur.2021.771034
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. https://doi.org/10.1093/cercor/bhs352
Dekhil O, Shalaby A, Soliman A, Mahmoud A, Kong M, Barnes G, Elmaghraby A, El-Baz A (2021) Identifying brain areas correlated with ADOS raw scores by studying altered dynamic functional connectivity patterns. Med Image Anal 68:101899. https://doi.org/10.1016/j.media.2020.101899
Rosso C, Moulton EJ, Kemlin C, Leder S, Corvol JC, Mehdi S, Obadia MA, Obadia M, Yger M, Meseguer E, Perlbarg V, Valabregue R, Magno S, Lindberg P, Meunier S, Lamy JC (2022) Cerebello-motor paired associative stimulation and motor recovery in stroke: a randomized, sham-controlled, double-blind pilot trial. Neurotherapeutics 19(2):491–500. https://doi.org/10.1007/s13311-022-01205-y
Tosun A, Türe S, Askin A, Yardimci EU, Demirdal SU, Kurt IT, Tosun O, Kocyigit H, Akhan G, Gelal FM (2017) Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil 24(5):361–367. https://doi.org/10.1080/10749357.2017.1305644
Zhang L, Xing G, Fan Y, Guo Z, Chen H, Mu Q (2017) Short- and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis. Clin Rehabil 31(9):1137–1153. https://doi.org/10.1177/0269215517692386
Xiang H, Sun J, Tang X, Zeng K, Wu X (2019) The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 33(5):847–864. https://doi.org/10.1177/0269215519829897
Fan H, Song Y, Cen X, Yu P, Bíró I, Gu Y (2021) The effect of repetitive transcranial magnetic stimulation on lower-limb motor ability in stroke patients: a systematic review. Front Hum Neurosci 15:620573. https://doi.org/10.3389/fnhum.2021.620573
Gao B, Wang Y, Zhang D, Wang Z, Wang Z (2022) Intermittent theta-burst stimulation with physical exercise improves poststroke motor function: a systemic review and meta-analysis. Front Neurol 13:964627. https://doi.org/10.3389/fneur.2022.964627
Liepert J, Hamzei F, Weiller C (2000) Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 23(11):1761–1763. https://doi.org/10.1002/1097-4598(200011)23:11%3c1761::aid-mus14%3e3.0.co;2-m
Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM (2002) Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125(Pt 8):1896–1907. https://doi.org/10.1093/brain/awf183
Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM (2003) Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke 34(11):2653–2658. https://doi.org/10.1161/01.STR.0000092122.96722.72
Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55(3):400–409. https://doi.org/10.1002/ana.10848
Koski L, Mernar TJ, Dobkin BH (2004) Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair 18(4):230–249. https://doi.org/10.1177/1545968304269210
Lin YL, Potter-Baker KA, Cunningham DA, Li M, Sankarasubramanian V, Lee J, Jones S, Sakaie K, Wang X, Machado AG, Plow EB (2020) Stratifying chronic stroke patients based on the influence of contralesional motor cortices: an inter-hemispheric inhibition study. Clin Neurophysiol 131(10):2516–2525. https://doi.org/10.1016/j.clinph.2020.06.016
Bocci T, Pietrasanta M, Cerri C, Restani L, Caleo M, Sartucci F (2014) Visual callosal connections: role in visual processing in health and disease. Rev Neurosci 25(1):113–127. https://doi.org/10.1515/revneuro-2013-0025
Bocci T, Nasini F, Caleo M, Restani L, Barloscio D, Ardolino G, Priori A, Maffei L, Nardi M, Sartucci F (2018) Unilateral application of cathodal tDCS reduces transcallosal inhibition and improves visual acuity in amblyopic patients. Front Behav Neurosci 12:109. https://doi.org/10.3389/fnbeh.2018.00109
Wang Q, Zhang D, Zhao Y, Hai H, Ma Y (2020) Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: a randomized clinical trial. Brain Stimul 13:979–986. https://doi.org/10.1016/j.brs.2020.03.020
Grefkes C, Fink GR (2012) Disruption of motor network connectivity post-stroke and its noninvasive neuromodulation. Curr Opin Neurol 25(6):670–675. https://doi.org/10.1097/WCO.0b013e3283598473
Paul T, Hensel L, Rehme AK, Tscherpel C, Eickhoff SB, Fink GR, Grefkes C, Volz LJ (2021) Early motor network connectivity after stroke: an interplay of general reorganization and state-specific compensation. Hum Brain Mapp 42(16):5230–5243. https://doi.org/10.1002/hbm.25612
Olafson ER, Jamison KW, Sweeney EM, Liu H, Wang D, Bruss JE, Boes AD, Kuceyeski A (2021) Functional connectome reorganization relates to post-stroke motor recovery and structural and functional disconnection. Neuroimage 245:118642. https://doi.org/10.1016/j.neuroimage.2021.118642
Hartwigsen G, Volz LJ (2021) Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. Neuroimage 224:117449. https://doi.org/10.1016/j.neuroimage.2020.117449
Binder E, Leimbach M, Pool EM, Volz LJ, Eickhoff SB, Fink GR, Grefkes C (2021) Cortical reorganization after motor stroke: a pilot study on differences between the upper and lower limbs. Hum Brain Mapp 42(4):1013–1033. https://doi.org/10.1002/hbm.25275
Rogers BP, Morgan VL, Newton AT, Gore JC (2007) Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25(10):1347–1357. https://doi.org/10.1016/j.mri.2007.03.007
van den Heuvel MP, Hulshoff PH (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20(8):519–534. https://doi.org/10.1016/j.euroneuro.2010.03.008
Desowska A, Turner DL (2019) Dynamics of brain connectivity after stroke. Rev Neurosci 30(6):605–623. https://doi.org/10.1515/revneuro-2018-0082
Xia Y, Huang G, Quan X, Qin Q, Li H, Xu C, Liang Z (2021) Dynamic structural and functional reorganizations following motor stroke. Med Sci Monit 27:e929092. https://doi.org/10.12659/MSM.929092
Volz LJ, Rehme AK, Michely J, Nettekoven C, Eickhoff SB, Fink GR, Grefkes C (2016) Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex 26(6):2882–2894. https://doi.org/10.1093/cercor/bhw034
Li S, Francisco GE, Rymer WZ (2021) A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair 35(7):601–610. https://doi.org/10.1177/15459683211011214
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
None.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Z., Liu, T., Han, K. et al. The effects of rTMS on motor recovery after stroke: a systematic review of fMRI studies. Neurol Sci 45, 897–909 (2024). https://doi.org/10.1007/s10072-023-07123-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07123-x