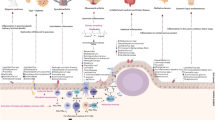
Abstract
Myasthenia gravis (MG) is a classic autoimmune neuromuscular disease with strong clinical heterogeneity. The concept of subgroup classification was proposed to guide the precise treatment of MG. Subgroups based on serum antibodies and clinical features include ocular MG, early-onset MG with AchR antibodies, late-onset MG with AchR antibodies, thymoma-associated MG, MuSK-associated MG, LRP4-associated MG, and seronegative MG. However, reliable objective biomarkers are still needed to reflect the individualized response to therapy. MicroRNAs (miRNAs) are small non-coding RNA molecules which can specifically bind to target genes and regulate gene expression at the post-transcriptional level, and then influence celluar biological processes. MiRNAs play an important role in the pathogenesis of autoimmune diseases, including MG. Several studies on circulating miRNAs in MG have been reported. However, there is rare systematic review to summarize the differences of these miRNAs in different subgroups of MG. Here, we summarize the potential role of circulating miRNAs in different subgroups of MG to promote personalized medicine.

Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Gilhus NE (2016) Myasthenia gravis. N Engl J Med 375(26):2570–2581
Mantegazza R, Bernasconi P, Cavalcante P (2018) Myasthenia gravis: from autoantibodies to therapy. Curr Opin Neurol 31(5):517–525
Gilhus NE, Skeie GO, Romi F et al (2016) Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol 12(5):259–268
Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14(10):1023–1036
Meriggioli MN, Sanders DB (2009) Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 8(5):475–490
Bhaskaran M, Mohan M (2014) MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol 51(4):759–774
Kim D, Chang HR, Baek D (2017) Rules for functional microRNA targeting. BMB Rep 50(11):554–559
Seitz H (2009) Redefining microRNA targets. Curr Biol 19(10):870–873
Winter J, Jung S, Keller S et al (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234
Yu AM, Choi YH, Tu MJ (2020) RNA drugs and RNA targets for small molecules: principles, progress, and challenges. Pharmacol Rev 72(4):862–898
Zhang L, Wu H, Zhao M et al (2020) Clinical significance of miRNAs in autoimmunity. J Autoimmun 109:102438
Zhang Y, Guo M, Xin N et al (2016) Decreased microRNA miR-181c expression in peripheral blood mononuclear cells correlates with elevated serum levels of IL-7 and IL-17 in patients with myasthenia gravis. Clin Exp Med 16(3):413–421
Liu XF, Wang RQ, Hu B et al (2016) MiR-15a contributes abnormal immune response in myasthenia gravis by targeting CXCL10. Clin Immunol 164:106–113
Cheng Z, Qiu S, Jiang L et al (2013) MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1. J Clin Immunol 33(3):567–576
Lu J, Yan M, Wang Y et al (2013) Altered expression of miR-146a in myasthenia gravis. Neurosci Lett 555:85–90
Cavalcante P, Mizrachi T, Barzago C et al (2019) MicroRNA signature associated with treatment response in myasthenia gravis: a further step towards precision medicine. Pharmacol Res 148:104388
Fortin E, Cestari DM, Weinberg DH (2018) Ocular myasthenia gravis: an update on diagnosis and treatment. Curr Opin Ophthalmol 29(6):477–484
Wong SH, Plant GT, Cornblath W (2016) Does treatment of ocular myasthenia gravis with early immunosuppressive therapy prevent secondarily generalization and should it be offered to all such patients? J Neuroophthalmol 36(1):98–102
Sabre L, Maddison P, Wong SH et al (2019) miR-30e-5p as predictor of generalization in ocular myasthenia gravis. Ann Clin Transl Neurol 6(2):243–251
Cheng T, Ding S, Liu S et al (2021) Resolvin D1 improves the Treg/Th17 imbalance in systemic lupus erythematosus through miR-30e-5p. Front Immunol 12:668760
Li Y, Yu J, Wang F et al (2021) MiR-150-5p regulate T cell activation in severe aplastic anemia by targeting Bach2. Cell Tissue Res 384(2):423–434
Ma Z, Shen Y, Zeng Q et al (2018) MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis. Int Immunopharmacol 54:188–197
Chunjie N, Huijuan N, Zhao Y et al (2015) Disease-specific signature of serum miR-20b and its targets IL-8 and IL-25, in myasthenia gravis patients. Eur Cytokine Netw 26(3):61–66
Molin CJ, Sabre L, Weis CA et al (2018) Thymectomy lowers the myasthenia gravis biomarker miR-150-5p. Neurol Neuroimmunol Neuroinflamm 5(3):e450
Barzago C, Lum J, Cavalcante P et al (2016) A novel infection- and inflammation-associated molecular signature in peripheral blood of myasthenia gravis patients. Immunobiology 221(11):1227–1236
Sabre L, Maddison P, Sadalage G (2018) Circulating microRNA miR-21-5p, miR-150-5p and miR-30e-5p correlate with clinical status in late onset myasthenia gravis. J Neuroimmunol 321:164–170
Nogales-Gadea G, Ramos-Fransi A, Suárez-Calvet X et al (2014) Analysis of serum miRNA profiles of myasthenia gravis patients. PLoS ONE 9(3):e91927
Punga T, Bartoccioni E, Lewandowska M et al (2016) Disease specific enrichment of circulating let-7 family microRNA in MuSK+ myasthenia gravis. J Neuroimmunol 292:21–26
Sabre L, Guptill JT, Russo M et al (2018) Circulating microRNA plasma profile in MuSK+ myasthenia gravis. J Neuroimmunol 325:87–91
Tan Y, Zhu L, Cui L, Guan Y (2021) Differential expression of miRNA in the peripheral blood mononuclear cells in myasthenia gravis with muscle-specific receptor tyrosine kinase antibodies. Crit Rev Eukaryot Gene Expr 31(2):1–15
Li J, Qiu D, Chen Z et al (2016) Altered expression of miR-125a-5p in thymoma-associated myasthenia gravis and its down-regulation of foxp3 expression in Jurkat cells. Immunol Lett 172:47–55
Wang Z, Chen Y, Xu S et al (2015) Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas. J Neuroimmunol 288:34–39
Xin Y, Cai H, Lu T et al (2016) miR-20b inhibits T cell proliferation and activation via NFAT signaling pathway in thymoma-associated myasthenia gravis. Biomed Res Int 2016:9595718
Somnier FE, Keiding N, Paulson OB (1991) Epidemiology of myasthenia gravis in Denmark. A longitudinal and comprehensive population survey. Arch Neurol 48(7):733–739
Fan L, Ma S, Yang Y et al (2019) Clinical differences of early and late-onset myasthenia gravis in 985 patients. Neurol Res 41(1):45–51
Santos E, Bettencourt A, da Silva AM et al (2017) HLA and age of onset in myasthenia gravis. Neuromuscul Disord 27(7):650–654
Barbaud A, Carlander B, Pagès M (2006) Formes tardives de myasthénie. Etude comparative avec la myasthénie du sujet jeune [Late onset forms of myasthenia gravis. Comparison with early-onset myasthenia gravis]. Rev Neurol (Paris) 162(10):990–996
Suzuki S, Utsugisawa K, Nagane Y et al (2011) Clinical and immunological differences between early and late-onset myasthenia gravis in Japan. J Neuroimmunol 230(1–2):148–152
Cron MA, Maillard S, Villegas J et al (2018) Thymus involvement in early-onset myasthenia gravis. Ann N Y Acad Sci 1412(1):137–145
Klimiec-Moskal E, Quirke M, Leite MI (2022) Comorbidities in older patients with myasthenia gravis-comparison between early- and late-onset disease. Acta Neurol Scand 145(3):371–374
Wolfe GI, Kaminski HJ, Aban IB et al (2017) Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 376(21):2097
Narayanaswami P, Sanders DB, Wolfe G et al (2021) International consensus guidance for management of Myasthenia Gravis: 2020 update. Neurology 96(3):114–122
Wolfe GI, Kaminski HJ, Aban IB et al (2019) Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 18(3):259–268
Alqarni F, Almalki D, Aljohani Z et al (2021) Prevalence and risk factors of myasthenia gravis recurrence post-thymectomy. Neurosciences (Riyadh) 26(1):4–14
Romano G, Zirafa CC, Ceccarelli I et al (2021) Robotic thymectomy for thymoma in patients with myasthenia gravis: neurological and oncological outcomes. Eur J Cardiothorac Surg 60(4):890–895
Punga T, Le Panse R, Andersson M et al (2014) Circulating miRNAs in myasthenia gravis: miR-150-5p as a new potential biomarker. Ann Clin Transl Neurol 1(1):49–58
Cron MA, Maillard S, Truffault F et al (2019) Causes and consequences of miR-150-5p dysregulation in myasthenia gravis. Front Immunol 10:539
Bortone F, Scandiffio L, Marcuzzo S et al (2020) miR-146a in myasthenia gravis thymus bridges innate immunity with autoimmunity and is linked to therapeutic effects of corticosteroids. Front Immunol 11:142
Saravanan S, Islam VI, Thirugnanasambantham K, Sekar D (2016) In silico identification of human miR 3654 and its targets revealed its involvement in prostate cancer progression. Microrna 5(2):140–145
Liu Y, Hu L, Liu Q et al (2022) miR-3651 participates in the growth cycle of hepatocellular carcinoma cells and promotes the malignant metastasis via the PI3K/AKT/mTOR signalling pathway. J Oncol 2022:5744999
Li H (2022) Physiologic and pathophysiologic roles of AKAP12. Sci Prog 105(3):368504221109212
Cowden JM, Yu F, Banie H et al (2014) The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann Rheum Dis 73(3):600–608
Saravanan C, Bharti SK, Jaggi S et al (2011) Histamine H4 receptor: a novel target for inflammation therapy. Mini Rev Med Chem 11(2):143–158
Chen J, Tian DC, Zhang C et al (2020) Incidence, mortality, and economic burden of myasthenia gravis in China: a nationwide population-based study. Lancet Reg Health West Pac 5:100063
Fiorillo AA, Heier CR, Huang YF et al (2020) Estrogen receptor, inflammatory, and FOXO transcription factors regulate expression of myasthenia gravis-associated circulating microRNAs. Front Immunol 11:151
Punga AR, Andersson M, Alimohammadi M, Punga T (2015) Disease specific signature of circulating miR-150-5p and miR-21-5p in myasthenia gravis patients. J Neurol Sci 356(1–2):90–96
Smigielska-Czepiel K, van den Berg A, Jellema P et al (2013) Dual role of miR-21 in CD4+ T-cells: activation-induced miR-21 supports survival of memory T-cells and regulates CCR7 expression in naive T-cells. PLoS ONE 8(10):e76217
Beretta F, Huang YF, Punga AR (2022) Towards personalized medicine in myasthenia gravis: role of circulating microRNAs miR-30e-5p, miR-150-5p and miR-21-5p. Cells 11(4):740
Belver L, de Yébenes VG, Ramiro AR (2010) MicroRNAs prevent the generation of autoreactive antibodies. Immunity 33(5):713–722
Tsai WC, Hsu PW, Lai TC et al (2009) MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 49(5):1571–1582
Märklin M, Heitmann JS, Kauer J et al (2020) Genetic loss of NFAT2 (NFATc1) impairs B cell development of B1 and B2 B cells. Cell Immunol 349:104048
Hock M, Vaeth M, Rudolf R et al (2013) NFATc1 induction in peripheral T and B lymphocytes. J Immunol 190(5):2345–2353
Yeh JH, Chen WH, Chiu HC et al (2004) Low frequency of MuSK antibody in generalized seronegative myasthenia gravis among Chinese. Neurology 62(11):2131–2132
Huang YC, Yeh JH, Chiu HC et al (2008) Clinical characteristics of MuSK antibody-positive myasthenia gravis in Taiwan. J Formos Med Assoc 107(7):572–575
Evoli A, Alboini PE, Damato V et al (2018) Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci 1412(1):82–89
Huijbers MG, Zhang W, Klooster R et al (2013) MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc Natl Acad Sci USA 110(51):20783–20788
Fichtner ML, Jiang R, Bourke A et al (2020) Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Front Immunol 11:776
Wang K, Yuan Y, Cho JH et al (2012) Comparing the microRNA spectrum between serum and plasma. PLoS ONE 7(7):e41561
Zhong H, Zhao C, Luo S (2019) HLA in myasthenia gravis: from superficial correlation to underlying mechanism. Autoimmun Rev 18(9):102349
Kurd N, Robey EA (2016) T-cell selection in the thymus: a spatial and temporal perspective. Immunol Rev 271(1):114–126
Nitta T, Takayanagi H (2021) Non-epithelial thymic stromal cells: unsung heroes in thymus organogenesis and T cell development. Front Immunol 11:620894
Ma D, Wei Y, Liu F (2013) Regulatory mechanisms of thymus and T cell development. Dev Comp Immunol 39(1–2):91–102
Sommer N, Willcox N, Harcourt GC, Newsom-Davis J (1990) Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol 28(3):312–319
Marx A, Porubsky S, Belharazem D (2015) Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol 270:55–65
Nakajima J, Murakawa T, Fukami T et al (2008) Postthymectomy myasthenia gravis: relationship with thymoma and antiacetylcholine receptor antibody. Ann Thorac Surg 86(3):941–945
Funding
This work was supported by The Open Project of Key Laboratory of Colleges and Universities in Jiangsu Province (XZSYSKF2021041), Science and Technology Development Fund of Affiliated Hospital of Xuzhou Medical University (XYFM2021017), Medical research project of Jiangsu Provincial Health Commission (M2022118), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_1270, SJCX22_1289).
Author information
Authors and Affiliations
Contributions
X. Y. H. and Z. A. Z. conceptualized the study and wrote the manuscript. Y. Y. W., M. M. X., and X. D. participated in the production of figure and table. Y. Z. contributed to edit a drafting of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
None.
Informed consent
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Zhang, Z., Wang, Y. et al. Circulating miRNAs drive personalized medicine based on subgroup classification in myasthenia gravis patients. Neurol Sci 44, 3877–3884 (2023). https://doi.org/10.1007/s10072-023-06933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06933-3