Abstract
Background
Dementia affects more than 55 million people worldwide. Several technologies have been developed to slow cognitive decline: deep brain stimulation (DBS) of network targets in Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) have been recently investigated.
Objective
This study aimed to review the characteristics of the populations, protocols, and outcomes of patients with dementia enrolled in clinical trials investigating the feasibility and efficacy of DBS.
Materials and methods
A systematic search of all registered RCTs was performed on Clinicaltrials.gov and EudraCT, while a systematic literature review was conducted on PubMed, Scopus, Cochrane, and APA PsycInfo to identify published trials.
Results
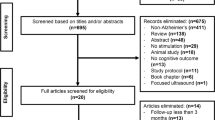
The literature search yielded 2122 records, and the clinical trial search 15 records. Overall, 17 studies were included. Two of 17 studies were open-label studies reporting no NCT/EUCT code and were analysed separately. Of 12 studies investigating the role of DBS in AD, we included 5 published RCTs, 2 unregistered open-label (OL) studies, 3 recruiting studies, and 2 unpublished trials with no evidence of completion. The overall risk of bias was assessed as moderate-high. Our review showed significant heterogeneity in the recruited populations regarding age, disease severity, informed consent availability, inclusion, and exclusion criteria. Notably, the standard mean of overall severe adverse events was moderately high (SAEs: 9.10 ± 7.10%).
Conclusion
The population investigated is small and heterogeneous, published results from clinical trials are under-represented, severe adverse events not negligible, and cognitive outcomes uncertain. Overall, the validity of these studies requires confirmation based on forthcoming higher-quality clinical trials.


Similar content being viewed by others
References
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031. https://doi.org/10.1016/S0140-6736(10)61349-9
Kurkinen M, Tran L (2021) Aduhelm: the best hope for Alzheimer’s disease patients or the worst decision the FDA has ever made? J Alzheimers Dis 84:969–971. https://doi.org/10.3233/JAD-215105
Hoy SM (2023) Lecanemab: first approval. Drugs 83:359–365. https://doi.org/10.1007/s40265-023-01851-2
Sugiyama K, Nozaki T, Asakawa T et al (2015) The present indication and future of deep brain stimulation. Neurol Med Chir (Tokyo) 55:416–421. https://doi.org/10.2176/NMC.RA.2014-0394
Kahn L, Sutton B, Winston HR et al (2021) Deep brain stimulation for obsessive-compulsive disorder: real world experience post-FDA-humanitarian use device approval. Front Psych 12:238. https://doi.org/10.3389/FPSYT.2021.568932/BIBTEX
Cleary DR, Ozpinar A, Raslan AM, Ko AL (2015) Deep brain stimulation for psychiatric disorders: where we are now. Neurosurg Focus 38:E2. https://doi.org/10.3171/2015.3.FOCUS1546
Yamamoto T, Katayama Y, Kobayashi K et al (2010) Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci 32:1145–1151. https://doi.org/10.1111/J.1460-9568.2010.07412.X
Boccard SGJ, Pereira EAC, Aziz TZ (2015) Deep brain stimulation for chronic pain. J Clin Neurosci 22:1537–1543. https://doi.org/10.1016/J.JOCN.2015.04.005
Bronte-Stewart H, Taira T, Valldeoriola F et al (2011) Inclusion and exclusion criteria for DBS in dystonia. Mov Disord 26(Suppl 1):S5–S16. https://doi.org/10.1002/MDS.23482
Della Flora E, Perera CL, Cameron AL, Maddern GJ (2010) Deep brain stimulation for essential tremor: a systematic review. Mov Disord 25:1550–1559. https://doi.org/10.1002/MDS.23195
Sun F, Zhang X, Dong S et al (2022) Effectiveness of low-frequency pallidal deep brain stimulation at 65 Hz in Tourette syndrome. Neuromodulation 25:286–295. https://doi.org/10.1111/NER.13456
Hardenacke K, Kuhn J, Lenartz D et al (2013) Stimulate or degenerate: deep brain stimulation of the nucleus basalis Meynert in Alzheimer dementia. World Neurosurg 80:S27.e35–S27.e43. https://doi.org/10.1016/J.WNEU.2012.12.005
Nazmuddin M, IHCHM P, van Laar T (2021) Electrical stimulation of the nucleus basalis of Meynert: a systematic review of preclinical and clinical data. Sci Rep 11(1):11751. https://doi.org/10.1038/s41598-021-91391-0
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/BMJ.N71
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/BMJ.D5928
Newcombe RG, Altman DG (2000) Proportions and their differences. Stat with Confid:45–57
Smith GS, Laxton AW, Tang-Wai DF et al (2012) Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol 69:1141–1148. https://doi.org/10.1001/ARCHNEUROL.2012.590
Kuhn J, Hardenacke K, Lenartz D et al (2015) Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol Psychiatry 20:353–360. https://doi.org/10.1038/MP.2014.32
Maltête D, Wallon D, Bourilhon J et al (2021) Nucleus basalis of Meynert stimulation for Lewy body dementia. Neurology 96:e684–e697. https://doi.org/10.1212/WNL.0000000000011227
Scharre DW, Weichart E, Nielson D et al (2018) Deep brain stimulation of frontal lobe networks to treat Alzheimer’s disease. J Alzheimers Dis 62:621–633. https://doi.org/10.3233/JAD-170082
Ponce FA, Asaad WF, Foote KD et al (2016) Bilateral deep brain stimulation of the fornix for Alzheimer’s disease: surgical safety in the ADvance trial. J Neurosurg 125:75–84. https://doi.org/10.3171/2015.6.JNS15716
Holroyd KB, Fosdick L, Smith GS et al (2015) Deep brain stimulation targeting the fornix for mild Alzheimer dementia: design of the ADvance randomized controlled trial. Open Access J Clin Trials 7:63–76. https://doi.org/10.2147/OAJCT.S81542
Leoutsakos JMS, Yan H, Anderson WS et al (2018) Deep brain stimulation targeting the fornix for mild Alzheimer dementia (the ADvance trial): a two year follow-up including results of delayed activation. J Alzheimers Dis 64:597–606. https://doi.org/10.3233/JAD-180121
Targum SD, Fosdick L, Drake KE et al (2021) Effect of age on clinical trial outcome in participants with probable Alzheimer’s disease. J Alzheimers Dis 82:1243–1257. https://doi.org/10.3233/JAD-210530
Lozano AM, Fosdick L, Chakravarty MM et al (2016) A phase II study of fornix deep brain stimulation in mild Alzheimer’s disease. J Alzheimers Dis 54:777–787. https://doi.org/10.3233/JAD-160017
Gratwicke J, Oswal A, Akram H et al (2020) Resting state activity and connectivity of the nucleus basalis of Meynert and globus pallidus in Lewy body dementia and Parkinson’s disease dementia. Neuroimage 221:117184. https://doi.org/10.1016/J.NEUROIMAGE.2020.117184
Mao ZQ, Wang X, Xu X et al (2018) Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation. Neural Regen Res 13:2164. https://doi.org/10.4103/1673-5374.241468
Noreik M, Kuhn J, Hardenacke K et al (2015) Changes in nutritional status after deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s disease — results of a phase I study. J Nutr Health Aging 19:812–818. https://doi.org/10.1007/S12603-015-0595-8
Baldermann JC, Hardenacke K, Hu X et al (2018) Neuroanatomical characteristics associated with response to deep brain stimulation of the nucleus basalis of Meynert for Alzheimer’s disease. Neuromodulation 21:184–190. https://doi.org/10.1111/NER.12626
Sankar T, Chakravarty MM, Bescos A et al (2015) Deep brain stimulation influences brain structure in Alzheimer’s disease. Brain Stimul 8:645–654. https://doi.org/10.1016/J.BRS.2014.11.020
Germann J, Elias GJB, Boutet A et al (2021) Brain structures and networks responsible for stimulation-induced memory flashbacks during forniceal deep brain stimulation for Alzheimer’s disease. Alzheimers Dement 17:777–787. https://doi.org/10.1002/ALZ.12238
Gratwicke J, Zrinzo L, Kahan J et al (2020) Bilateral nucleus basalis of Meynert deep brain stimulation for dementia with Lewy bodies: a randomised clinical trial. Brain Stimul 13:1031–1039. https://doi.org/10.1016/J.BRS.2020.04.010
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–939. https://doi.org/10.1212/WNL.34.7.939
Frisoni GB, Winblad B, O’Brien JT (2011) Revised NIA-AA criteria for the diagnosis of Alzheimer’s disease: a step forward but not yet ready for widespread clinical use. Int Psychogeriatr 23:1191–1196. https://doi.org/10.1017/S1041610211001220
Dubois B, Feldman HH, Jacova C et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13:614–629. https://doi.org/10.1016/S1474-4422(14)70090-0
Mckeith IG (2006) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 66:1455; author reply 1455. https://doi.org/10.1212/01.wnl.0000224698.67660.45
Stratmann K, Heinsen H, Korf HW et al (2016) Precortical phase of Alzheimer’s disease (AD)-related tau cytoskeletal pathology. Brain Pathol 26:371–386. https://doi.org/10.1111/BPA.12289/SUPPINFO
Luo Y, Sun Y, Tian X et al (2021) Deep brain stimulation for Alzheimer’s disease: stimulation parameters and potential mechanisms of action. Front Aging Neurosci 13:104. https://doi.org/10.3389/FNAGI.2021.619543/BIBTEX
Leplus A, Lauritzen I, Melon C et al (2019) Chronic fornix deep brain stimulation in a transgenic Alzheimer’s rat model reduces amyloid burden, inflammation, and neuronal loss. Brain Struct Funct 224:363–372. https://doi.org/10.1007/S00429-018-1779-X
Mesulam M-M, Geula C (1988) Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol 275:216–240. https://doi.org/10.1002/CNE.902750205
Kumbhare D, Palys V, Toms J et al (2018) Nucleus basalis of Meynert stimulation for dementia: theoretical and technical considerations. Front Neurosci 12:3. https://doi.org/10.3389/FNINS.2018.00614/FULL
Bittlinger M (2018) Müller S (2018) Opening the debate on deep brain stimulation for Alzheimer disease — a critical evaluation of rationale, shortcomings, and ethical justification. BMC Med Ethics 191(19):1–23. https://doi.org/10.1186/S12910-018-0275-4
Reitz C, Rogaeva E, Beecham GW (2020) Late-onset vs nonmendelian early-onset Alzheimer disease. Neurol Genet 6:e512. https://doi.org/10.1212/NXG.0000000000000512
Koedam ELGE, Pijnenburg YAL, Deeg DJH et al (2008) Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord 26:147–152. https://doi.org/10.1159/000149585
Stanley K, Walker Z (2014) Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr 26:1945–1953. https://doi.org/10.1017/s1041610214001173
Murray ME, Graff-Radford NR, Ross OA et al (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10(9):785–796. https://doi.org/10.1016/S1474-4422(11)70156-9
Stanley K, Whitfield T, Kuchenbaecker K et al (2019) Rate of cognitive decline in Alzheimer’s disease stratified by age. J Alzheimers Dis 69:1153–1160. https://doi.org/10.3233/JAD-181047
Clausen J (2010) Ethical brain stimulation — neuroethics of deep brain stimulation in research and clinical practice. Eur J Neurosci 32:1152–1162. https://doi.org/10.1111/J.1460-9568.2010.07421.X
Mak E, Chin R, Ng LT et al (2015) Clinical associations of anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry 30:1207–1214. https://doi.org/10.1002/GPS.4275
Grant RA, Halpern CH, Baltuch GH et al (2014) Ethical considerations in deep brain stimulation for psychiatric illness. J Clin Neurosci 21:1–5. https://doi.org/10.1016/j.jocn.2013.04.004
Siegel AM, Barrett MS, Bhati MT (2017) Deep brain stimulation for Alzheimer’s disease: ethical challenges for clinical research. J Alzheimers Dis 56:429–439. https://doi.org/10.3233/JAD-160356
Laxton AW, Tang-Wai DF, McAndrews MP et al (2010) A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann Neurol 68:521–534. https://doi.org/10.1002/ANA.22089
Gillette-Guyonnet S, Andrieu S, Nourhashemi F et al (2011) Long-term progression of Alzheimer’s disease in patients under antidementia drugs. Alzheimers Dement 7:579–592. https://doi.org/10.1016/J.JALZ.2011.02.009
Ito K, Ahadieh S, Corrigan B et al (2010) Disease progression meta-analysis model in Alzheimer’s disease. Alzheimers Dement 6:39–53. https://doi.org/10.1016/J.JALZ.2009.05.665
Conrado DJ, Denney WS, Chen D, Ito K (2014) An updated Alzheimer’s disease progression model: incorporating non-linearity, beta regression, and a third-level random effect in NONMEM. J Pharmacokinet Pharmacodyn 41:581–598. https://doi.org/10.1007/S10928-014-9375-Z
ADvance II Study: DBS-f in patients with mild Alzheimer’s disease — full text view — ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03622905. Accessed 27 Feb 2022
Author information
Authors and Affiliations
Contributions
Conceptualisation: G.R., L.T., N.V., E.L., F.S.; methodology: G.R., L.T., N.V.; formal analysis and investigation: G.R., L.T., P.M., F.S.; writing — original draft preparation: L.T., G.R.; writing — review and editing: I.A., C.F., F.D.R., M.C., E.L., G.B., M.L., N.V.; resources: N.V., M.L., I.A., C.F.; supervision: N.V., M.L.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Remoli, G., Tariciotti, L., Remore, L.G. et al. An updated overview of recent and ongoing deep brain stimulation (DBS) trials in patients with dementia: a systematic review. Neurol Sci 44, 3395–3427 (2023). https://doi.org/10.1007/s10072-023-06821-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06821-w