Abstract
Objectives
This study provides a systematic review and meta-analysis of randomized controlled trials (RCTs) investigating the safety and efficacy of lithium in amyotrophic lateral sclerosis (ALS) patients.
Methods
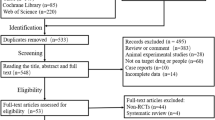
PubMed, Web of Science, Cochrane CENTRAL, Scopus, and Your Journals@Ovid were searched up to 9 December 2022. RCTs investigating lithium, either alone or with any supplement, in ALS patients were included. Meta-analysis was performed using RevMan and results are presented in forest plot.
Results
Four RCTs with 469 patients met the inclusion criteria and were included in our study. Lithium doses varied among the included studies and one study used a combined therapy of lithium with valproate. Meta-analysis showed no difference between lithium and placebo regarding severe adverse events (odds ratio = 1.13, 95% confidence interval: 0.73 to 1.75, P = 0.58). No significant differences were observed with regard to survival rate between the two groups (hazard ratio = 0.95, 95% confidence interval: 0.65 to 1.37, P = 0.77). There were also no significant differences between the two groups with regard to average changes of revised amyotrophic lateral sclerosis functional rating scale (P = 0.35) and forced vital capacity percentage predicted (P = 0.73). Subgroup analysis showed no significant differences regarding all investigated outcomes either for lithium alone or lithium with valproate.
Conclusion
Current evidence suggests a safety profile with no benefit of lithium for ALS. However, given the limited number of RCTs and the safety findings, we recommend further well-designed RCTs to investigate lithium and valproate in ALS patients.





Similar content being viewed by others
References
Hardiman O, Al-Chalabi A, Chio A et al (2017) Amyotrophic lateral sclerosis [published correction appears in Nat Rev Dis Primers. 2017 Oct 20;3:17085]. Nat Rev Dis Primers 3:17071. Published 2017 Oct 5. https://doi.org/10.1038/nrdp.2017.71
Mehta P, Horton DK, Kasarskis EJ et al (2017) CDC Grand Rounds: National Amyotrophic Lateral Sclerosis (ALS) Registry Impact, Challenges, and Future Directions [published correction appears in MMWR Morb Mortal Wkly Rep. 2018 Jan 19;67(2):81]. MMWR Morb Mortal Wkly Rep 66(50):1379–1382. Published 2017 Dec 22. https://doi.org/10.15585/mmwr.mm6650a3
Bettencourt C, Houlden H (2015) Exome sequencing uncovers hidden pathways in familial and sporadic ALS. Nat Neurosci 18(5):611–613. https://doi.org/10.1038/nn.4012
Brown CA, Lally C, Kupelian V, Flanders WD (2021) Estimated Prevalence and Incidence of Amyotrophic Lateral Sclerosis and SOD1 and C9orf72 Genetic Variants. Neuroepidemiology 55(5):342–353. https://doi.org/10.1159/000516752
Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 330(9):585–591. https://doi.org/10.1056/NEJM199403033300901
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996) Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347(9013):1425–1431. https://doi.org/10.1016/s0140-6736(96)91680-3
Writing Group; Edaravone (MCI-186) ALS 19 Study Group (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16(7):505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Paganoni S, Macklin EA, Hendrix S et al (2020) Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N Engl J Med 383(10):919–930. https://doi.org/10.1056/NEJMoa1916945
Nivoli AM, Colom F, Murru A et al (2011) New treatment guidelines for acute bipolar depression: a systematic review. J Affect Disord 129(1–3):14–26. https://doi.org/10.1016/j.jad.2010.05.018
Pasquali L, Busceti CL, Fulceri F, Paparelli A, Fornai F (2010) Intracellular pathways underlying the effects of lithium. Behav Pharmacol 21(5–6):473–492. https://doi.org/10.1097/FBP.0b013e32833da5da
Won E, Kim YK (2017) An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms. Int J Mol Sci. 18(12):2679. Published 2017 Dec 11. https://doi.org/10.3390/ijms18122679
Chalecka-Franaszek E, Chuang DM (1999) Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A 96(15):8745–8750. https://doi.org/10.1073/pnas.96.15.8745
Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM (2003) Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience 117(1):55–61. https://doi.org/10.1016/s0306-4522(02)00577-8
Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM (2002) Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 43(7):1173–1179. https://doi.org/10.1016/s0028-3908(02)00217-4
Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM (2008) Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience 155(3):567–572. https://doi.org/10.1016/j.neuroscience.2008.06.040
Dill J, Wang H, Zhou F, Li S (2008) Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci 28(36):8914–8928. https://doi.org/10.1523/JNEUROSCI.1178-08.2008
Vallée A, Vallée JN, Lecarpentier Y (2021) Parkinson's Disease: Potential Actions of Lithium by Targeting the WNT/β-Catenin Pathway, Oxidative Stress, Inflammation and Glutamatergic Pathway. Cells 10(2):230. Published 2021 Jan 25. https://doi.org/10.3390/cells10020230
Lazzara CA, Kim YH (2015) Potential application of lithium in Parkinson's and other neurodegenerative diseases. Front Neurosci 9:403. Published 2015 Oct 27. https://doi.org/10.3389/fnins.2015.00403
Fornai F, Longone P, Cafaro L et al (2008) Lithium delays progression of amyotrophic lateral sclerosis [published correction appears in Proc Natl Acad Sci U S A. 2008 Oct 21;105(42):16404–7]. Proc Natl Acad Sci U S A 105(6):2052–2057. https://doi.org/10.1073/pnas.0708022105
Aggarwal SP, Zinman L, Simpson E et al (2010) Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9(5):481–488. https://doi.org/10.1016/S1474-4422(10)70068-5
Boll MC, Alcaraz-Zubeldia M, Rios C, González-Esquivel D, Montes S (2022) A phase 2, double-blind, placebo-controlled trial of a valproate/lithium combination in ALS patients [published online ahead of print, 2022 Aug 29]. Neurologia (Engl Ed) S2173–5808(22)00089-X. https://doi.org/10.1016/j.nrleng.2022.07.003
Willemse SW, Roes KCB, Van Damme P et al (2022) Lithium carbonate in amyotrophic lateral sclerosis patients homozygous for the C-allele at SNP rs12608932 in UNC13A: protocol for a confirmatory, randomized, group-sequential, event-driven, double-blind, placebo-controlled trial. Trials 23(1):978. https://doi.org/10.1186/s13063-022-06906-5
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. Published 2009 Jul 21. https://doi.org/10.1136/bmj.b2535
Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299. https://doi.org/10.1080/146608200300079536
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210. Published 2016 Dec 5. https://doi.org/10.1186/s13643-016-0384-4
Altman DG, Bland JM (2011) How to obtain the confidence interval from a P value. BMJ 343:d2090. Published 2011 Aug 8. https://doi.org/10.1136/bmj.d2090
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. Published 2019 Aug 28. https://doi.org/10.1136/bmj.l4898
Verstraete E, Veldink JH, Huisman MH et al (2012) Lithium lacks effect on survival in amyotrophic lateral sclerosis: a phase IIb randomised sequential trial. J Neurol Neurosurg Psychiatry 83(5):557–564. https://doi.org/10.1136/jnnp-2011-302021
UKMND-LiCALS Study Group, Morrison KE, Dhariwal S et al (2013) Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial [published correction appears in Lancet Neurol. 2013 Sep;12(9):846]. Lancet Neurol 12(4):339–345. https://doi.org/10.1016/S1474-4422(13)70037-1
van Harten PN, Hoek HW, Matroos GE, van Os J (2008) Evidence that lithium protects against tardive dyskinesia: the Curaçao Extrapyramidal syndromes study VI. Eur Neuropsychopharmacol 18(2):152–155. https://doi.org/10.1016/j.euroneuro.2007.07.004
Straten G, Saur R, Laske C et al (2011) Influence of lithium treatment on GDNF serum and CSF concentrations in patients with early Alzheimer’s disease. Curr Alzheimer Res 8(8):853–859. https://doi.org/10.2174/156720511798192754
Berk M, Dandash O, Daglas R et al (2017) Neuroprotection after a first episode of mania: a randomized controlled maintenance trial comparing the effects of lithium and quetiapine on grey and white matter volume [published correction appears in Transl Psychiatry. 2017 Feb 21;7(2):e1041]. Transl Psychiatry 7(1):e1011. https://doi.org/10.1038/tp.2016.281
Miller RG, Moore DH, Forshew DA et al (2011) Phase II screening trial of lithium carbonate in amyotrophic lateral sclerosis: examining a more efficient trial design. Neurology 77(10):973–979. https://doi.org/10.1212/WNL.0b013e31822dc7a5
Wicks P, Vaughan TE, Massagli MP, Heywood J (2011) Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat Biotechnol 29(5):411–414. https://doi.org/10.1038/nbt.1837
Chiò A, Borghero G, Calvo A et al (2010) Lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurology 75(7):619–625. https://doi.org/10.1212/WNL.0b013e3181ed9e7c
van Eijk RPA, Jones AR, Sproviero W et al (2017) Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials [published correction appears in Neurology. 2017 Nov 28;89(22):2303]. Neurology 89(18):1915–1922. https://doi.org/10.1212/WNL.0000000000004606
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamad, A.A., Attia, A.N., Al-dardery, N.M. et al. Safety and efficacy of lithium in patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis of randomized controlled trials. Neurol Sci 44, 3029–3036 (2023). https://doi.org/10.1007/s10072-023-06814-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06814-9