Abstract
Objective
HIV-associated neurocognitive disorder (HAND) affects multiple cognitive domains and currently, the neuropsychological testing is the gold standard to identify these deficits. The aim of this longitudinal 12-month pilot study is to determine the effect of intensified combination antiretroviral therapy (cART) on rs-fMRI in virally suppressed (both in CSF and blood) patients with active HAND (those who have progressive neurocognitive impairment) and correlated with neurocognitive function tests.
Methods
In this pilot study, we have evaluated sixteen patients with active HAND with viral suppression in both blood and CSF to study the effect of cART on functional connectivity. Participants underwent rs-fMRI at the baseline (time point-1 (TP-1) and 12-month visits (time point-2 (TP-2)). Connectivity in the five major networks was measured at TP-1 and TP-2 using the seed-based approach. All the participants underwent a five-domain neuropsychological battery at TP-1 and TP-2. Neurocognitive scores (NC) as well as blood and CSF markers were correlated with functional connectivity (FC).
Results
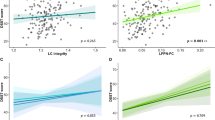
There was a significant increase in the FC between the two time points within the executive, salience, default mode, dorsal attention, and visual networks at voxel level threshold of p < 0.001 and cluster level threshold of p < 0.05 and corrected for false detection rate (FDR). The neurocognitive scores were positively correlated with all the networks at similar cluster and voxel level thresholds.
Conclusions
These results indicate that rs-fMRI can be potentially used as one of the biomarkers for treatment efficacy in HAND.



Similar content being viewed by others
Data Availability
Data is available and some data can be shared upon request.
References
Heaton R, Clifford D, Franklin D et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology 75(23):2087–2096
Cysique LA, Brew BJ (2011) Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 17(2):176–183
Tozzi V, Balestra P, Bellagamba R et al (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. JAIDS J Acquir Immune Defici Syndr 45(2):174–182
Chang L, Speck O, Miller EN et al (2001) Neural correlates of attention and working memory deficits in HIV patients. Neurology 57:1001–1007
Chang L, Yakupov R, Nakama H, Stokes B, Ernst T (2008) Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J NeuroImmune Pharmacol 3:95–104
Ernst T, Chang L, Jovicich J, Ames N, Arnold S (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59(9):1343–1349
Caldwell JZK, Gongvatana A, Navia BA et al (2014) Neural dysregulation during a working memory task in human immunodeficiency virus-seropositive and hepatitis C coinfected individuals. J Neurovirol 20:398–411
Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R (2014) HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS 28:803–811
Thames AD, Sayegh P, Terashima K et al (2016) Increased subcortical neural activity among HIV+ individuals during a lexical retrieval task. Neurobiology of disease 92:175–182
Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM (2013) Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology 80(13):1186–1193
Guha A, Wang L, Tanenbaum A et al (2016) Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. J Neurovirol 22(1):80–87
Wang X, Foryt P, Ochs R et al (2011) Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain connectivity 1(3):207–217
Ipser JC, Brown GG, Bischoff-Grethe A et al (2015) HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc 21(3):203–213
Ortega M, Brier MR, Ances BM (2015) Effects of HIV and combination antiretroviral therapy (cART) on cortico-striatal functional connectivity. AIDS 29(6):703
du Plessis L, Paul RH, Hoare J et al (2017) Resting-state functional magnetic resonance imaging in clade C HIV: within-group association with neurocognitive function. J Neurovirol 23(6):875–885
Ann HW, Jun S, Shin NY et al (2016) Characteristics of resting-state functional connectivity in HIV-associated neurocognitive disorder. PLoS ONE 11(4):e0153493
Chaganti JR, Heinecke A, Gates TM, Moffat KJ, Brew BJ (2017) Functional connectivity in virally suppressed patients with HIV-associated neurocognitive disorder: a resting-state analysis. AJNR Am J Neuroradiol 38(8):1623–1629
Zhuang Y, Qiu X, Wang L et al (2017) Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naïve HIV-infected individuals. J Neurovirol 23(5):704–712
Corrêa DG, Zimmermann N, Ventura N et al (2017) Longitudinal evaluation of resting-state connectivity, white matter integrity and cortical thickness in stable HIV infection: Preliminary results. Neuroradiol J 30(6):535–545
Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ (2016) Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 30(4):591–600
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2(3):125–41
Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37(1):90–101
Cole JH, Caan MWA, Underwood J, De Francesco D, van Zoest RA, Wit FWNM, Mutsaerts HJMM, Leech R, Geurtsen GJ, Portegies P, Majoie CBLM, Schim van der Loeff MF, Sabin CA, Reiss P, Winston A, Sharp DJ, Comorbidity in Relations to AIDS (COBRA) Collaboration (2018) No evidence for accelerated aging-related brain pathology in treated human immunodeficiency virus: longitudinal neuroimaging results from the comorbidity in relation to AIDS (COBRA) project. Clin Infect Dis. 66(12):1899–1909. https://doi.org/10.1093/cid/cix1124
Tanaka S, Manabu Honda M, Sadato N (2005) Modality-specific cognitive function of medial and lateral human Brodmann area 6. J Neurosci 25(2):496–501
Burgess PW, Wu H-C (2013) Rostral prefrontal cortex (Brodmann area 10): metacognition in the brain. In: Stuss DT, Knight RT (eds) Chapter 31: Principles of frontal lobe function. Oxford University Press, New York
Li R, Wang W, Wang Y, Peters S, Zhang X, Li H (2019) Effects of early HIV infection and combination antiretroviral therapy on intrinsic brain activity: a cross-sectional resting-state fMRI study. Neuropsychiatr Dis Treat 15:883–894
Ramirez-Mahaluf JP, Perramon J, Otal B et al (2018) Subgenual anterior cingulate cortex controls sadness-induced modulations of cognitive and emotional network hubs. Sci Rep 8:8566. https://doi.org/10.1038/s41598-018-26317-4
Menon V (2015) Salience network. In: Toga AW (ed) Brain mapping: an encyclopedic reference, vol 2. Elsevier Inc., London, UK, pp 597–611
Steven FL, Hurley RA, Hurley RA, Hayman AL, Taber KH (2011) Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 23(2):121–125
Janssen MAM, Hinne M, Janssen RJ et al (2017) Resting-state subcortical functional connectivity in HIV-infected patients on long-term cART. Brain Imaging Behav 11(5):1555–1560. https://doi.org/10.1007/s11682-016-9632-4
Sakurai Y (2017) Brodmann areas 39 and 40: human parietal association area and higher cortical function. Brain and nerve 69:461–469. https://doi.org/10.11477/mf.1416200765
Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM (2012) The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS 26(12):1501–1508
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and informed consent
This is a prospective longitudinal design (“The patient group was chosen from the study which was previously published in AIDS (2016, 30:591–600)”, at St Vincent’s Hospital (SVH), Sydney, Australia (ClinicalTrials.gov Identifier: NCT01449006).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaganti, J., Gates, T.M. & Brew, B.J. Reversible large-scale network disruption correlates with neurocognitive improvement in HIV-associated minor neurocognitive disorder with combined anti-retroviral therapy intensification: a prospective longitudinal resting-state functional magnetic resonance imaging study. Neurol Sci 44, 3261–3269 (2023). https://doi.org/10.1007/s10072-023-06783-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06783-z