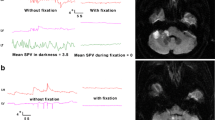
Abstract
Objectives
To investigate the frequency and pattern of horizontal saccadic dysmetria in unilateral cerebellar infarction and identify the responsible region for horizontal saccadic dysmetria.
Methods
From the acute stroke registry of Keimyung University Dongsan Medical Center between July 2016 and October 2020, 43 patients with acute unilateral cerebellar infarction were enrolled. Eye movements were recorded during the acute period and the lesion was mapped using MRIcron software for subtraction analysis.
Saccadic dysmetria was marked as hypometric when the gain is < 0.85 and hypermetric when > 1.0.
Results
Among the 43 participants, 30 patients (69.8%) demonstrated saccadic dysmetria. The age was significantly higher in patients with dysmetria (66.87 ± 12.82 vs. 53.54 ± 14.09, p = 0.004). Type of dysmetria showed a significant difference according to the vascular territory of the lesion. The posterior inferior cerebellar artery (PICA) infarction group presented ipsiversive saccadic dysmetria, while the superior cerebellar artery (SCA) group showed contraversive dysmetria (p < 0.001). In the SCA group, the culmen, fastigium, and dentate were the most frequently damaged regions, while the tonsil and inferior semilunar lobule were in the PICA group.
Conclusion
Saccadic dysmetria was observed in a large proportion of cerebellar stroke patients, and the types of saccades were distinctive according to the vascular territory of the lesion.

Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Thurtell MJ, Tomsak RL, Leigh RJ (2007) Disorders of saccades. Curr Neurol Neurosci Rep 7:407–416
Robinson FR, Fuchs AF (2001) The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24:981–1004
Beh SC, Frohman TC, Frohman EM (2017) Cerebellar control of eye movements. J Neuroophthalmol 37:87–98
Fujikado T, Noda H (1987) Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol 394:573–594
Goffart L, Chen LL, Sparks DL (2004) Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92:3351–3367
Ignashchenkova A, Dash S, Dicke PW, Haarmeier T, Glickstein M, Thier P (2009) Normal spatial attention but impaired saccades and visual motion perception after lesions of the monkey cerebellum. J Neurophysiol 102:3156–3168
Sato H, Noda H (1992) Saccadic dysmetria induced by transient functional decortication of the cerebellar vermis [corrected]. Exp Brain Res 88:455–458
Srivastava A, Ahmad OF, Pacia CP, Hallett M, Lungu C (2018) The relationship between saccades and locomotion. J Mov Disord 11:93–106
Choi KD, Kim HJ, Cho BM, Kim JS (2008) Saccadic adaptation in lateral medullary and cerebellar infarction. Exp Brain Res 188:475–482
Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H (2002) Human cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging study. Ophthalmologica 216:399–405
Tatu L, Moulin T, Bogousslavsky J, Duvernoy H (1996) Arterial territories of human brain: brainstem and cerebellum. Neurology 47:1125–1135
Sul B, Kim JS, Hong BY, Lee KB, Hwang WS, Kim YK et al (2016) The prognosis and recovery of aphasia related to stroke lesion. Ann Rehabil Med 40:786–793
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131
Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19:1081–1088
Bötzel K, Rottach K, Büttner U (1993) Normal and pathological saccadic dysmetria. Brain 116:337–353
Helmchen C, Hagenow A, Miesner J, Sprenger A, Rambold H, Wenzelburger R et al (2003) Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain 126:1319–1332
Moschner C, Perlman S, Baloh RW (1994) Comparison of oculomotor findings in the progressive ataxia syndromes. Brain 117(Pt 1):15–25
Wessel K, Moschner C, Wandinger KP, Kömpf D, Heide W (1998) Oculomotor testing in the differential diagnosis of degenerative ataxic disorders. Arch Neurol 55:949–956
Kim HA, Lee H, Yi HA, Lee SR, Lee SY, Baloh RW (2009) Pattern of otolith dysfunction in posterior inferior cerebellar artery territory cerebellar infarction. J Neurol Sci 280:65–70
King S, Chen AL, Joshi A, Serra A, Leigh RJ (2011) Effects of cerebellar disease on sequences of rapid eye movements. Vision Res 51:1064–1074
de Campos Netto AAT, Colafêmina JF (2010) Saccadic Movements in subjects with cerebellar disorders. Braz J Otorhinolaryngol 76:51–58
Ranalli PJ, Sharpe JA (1986) Contrapulsion of saccades and ipsilateral ataxia: a unilateral disorder of the rostral cerebellum. Ann Neurol 20:311–316
Shim DB, Song MH, Park KC, Song CE (2014) A case of pseudo-vestibular neuritis with contralesional canal paresis due to spontaneous bilateral vertebral artery dissection. Korean J Otorhinolaryngol-Head Neck Surg 57:552–555
Takagi M, Zee DS, Tamargo RJ (1998) Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80:1911–1931
Dieterich M, Bucher SF, Seelos KC, Brandt T (2000) Cerebellar activation during optokinetic stimulation and saccades. Neurology 54:148–155
Filippopulos F, Eggert T, Straube A (2013) Effects of cerebellar infarcts on cortical processing of saccades. J Neurol 260:805–814
Stephan T, Mascolo A, Yousry TA, Bense S, Brandt T, Dieterich M (2002) Changes in cerebellar activation pattern during two successive sequences of saccades. Hum Brain Mapp 16:63–70
Mano N, Ito Y, Shibutani H (1991) Saccade-related Purkinje cells in the cerebellar hemispheres of the monkey. Exp Brain Res 84:465–470
Ron S, Robinson DA (1973) Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36:1004–1022
Kheradmand A, Zee DS (2011) Cerebellum and ocular motor control. Front Neurol 2:53
Frohman EM, Frohman TC, Fleckenstein J, Racke MK, Hawker K, Kramer PD (2001) Ocular contrapulsion in multiple sclerosis: clinical features and pathophysiological mechanisms. J Neurol Neurosurg Psychiatry 70:688–692
Helmchen C, Straube A, Büttner U (1994) Saccadic lateropulsion in Wallenberg’s syndrome may be caused by a functional lesion of the fastigial nucleus. J Neurol 241:421–426
Tilikete C, Hermier M, Pelisson D, Vighetto A (2002) Saccadic lateropulsion and upbeat nystagmus: disorders of caudal medulla. Ann Neurol 52:658–662
Tilikete C, Koene A, Nighoghossian N, Vighetto A, Pélisson D (2006) Saccadic lateropulsion in Wallenberg syndrome: a window to access cerebellar control of saccades? Exp Brain Res 174:555–565
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sohyeon Kim and Hyun Ah Kim. The first draft of the manuscript was written by Sohyeon Kim, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. The study complied with the tenets of the Declaration of Helsinki. The protocol was reviewed by the Institutional Review Board (IRB) of the School of Medicine at Keimyung University.
Informed consent
Informed consent was waived by the IRB since the research involves no more than minimal risk to participants and does not adversely affect the rights and welfare of them.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Kim, H.A. & Lee, H. The frequency and characteristics of saccadic dysmetria in isolated cerebellar infarction. Neurol Sci 44, 2097–2102 (2023). https://doi.org/10.1007/s10072-023-06668-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06668-1