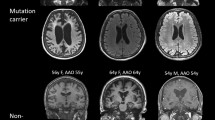
Abstract
Background
Alzheimer’s disease (AD) is a debilitating and highly heritable neurodegenerative disease. Early-onset AD (EOAD) was defined as AD occurring before age 65. Although it has a high genetic risk, EOAD due to PSEN2 variation is very rare. ABCA7 is an important risk gene for AD. Previously reported cases mainly carried variations in a single pathogenic or risk gene.
Methods and Results
In this study, we report a 35-year-old female carrying variants in both the PSEN2 gene (c.640G > T p.V214L) and ABCA7 gene (c.2848G > A p.V950M). Four previously reported cases carried PSEN2 V214L, and no reported cases carried ABCA7 V950M. She had a history of migraine, patent foramen ovale, spontaneous subarachnoid hemorrhage without aneurysm, and multiple cerebral microhemorrhages. Her MMSE score was 24/30, and her MoCA score was 22/30. The concentration of Aβ42 and the ratio of Aβ42 to Aβ40 in cerebral spinal fluid were obviously decreased. Published variants of PSEN2 and ABCA7 in PubMed were reviewed, and the patients’ characteristics were summarized and compared to provide information for the clinical diagnosis of AD.
Conclusions
It is necessary to conduct genetic screening in cases with atypical manifestations.



Similar content being viewed by others
Data availability
The raw data presented in this article will be available by the authors, and reasonable requests to access the datasets should be directed to the corresponding author after legal permission.
Code availability
Not applicable.
References
Scheltens P, De Strooper B, Kivipelto M et al (2021) Alzheimerʼs disease. Lancet 397:1577–1590
Uddin MS, Hasana S, Hossain MF et al (2021) Molecular Genetics of Early- and Late-Onset Alzheimer’s Disease. Curr Gene Ther 21:43–52
Kamboh MI (2022) Genomics and functional genomics of Alzheimerʼs disease. Neurotherapeutics 19:152–172
Ayodele T, Rogaeva E, Kurup JT, Beecham G, Reitz C (2021) Early-onset Alzheimerʼs disease: what is missing in research? Curr Neurol Neurosci Rep 21:4
Cacace R, Sleegers K, Van Broeckhoven C (2016) Molecular genetics of early-onset Alzheimerʼs disease revisited. Alzheimerʼs Dement 12:733–748
Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL (2007) Early-onset Alzheimerʼs disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol 20:29–33
Sperling RA, Aisen PS, Beckett LA et al (2011) Toward defining the preclinical stages of Alzheimerʼs disease: recommendations from the National Institute on Aging-Alzheimerʼs Association workgroups on diagnostic guidelines for Alzheimerʼs disease. Alzheimers Dement 7:280–292
Lanoiselée HM, Nicolas G, Wallon D et al (2017) APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med 14:e1002270
Yuksel M, Tacal O (2019) Trafficking and proteolytic processing of amyloid precursor protein and secretases in Alzheimer’s disease development: an up-to-date review. Eur J Pharmacol 856:172415
Nam H, Lee Y, Kim B et al (2022) Presenilin 2 N141I mutation induces hyperactive immune response through the epigenetic repression of REV-ERBα. Nat Commun 13:1972
Cai Y, An SS, Kim S (2015) Mutations in presenilin 2 and its implications in Alzheimerʼs disease and other dementia-associated disorders. Clin Interv Aging 10:1163–1172
Karch CM, Goate AM (2015) Alzheimerʼs disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77:43–51
Andrews SJ, Fulton-Howard B, Goate A (2020) Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol 19:326–335
De Roeck A, Van Broeckhoven C, Sleegers K (2019) The role of ABCA7 in Alzheimerʼs disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol 138:201–220
Dib S, Pahnke J, Gosselet F (2021) Role of ABCA7 in human health and in Alzheimerʼs disease. Int J Mol Sci 22(9):4603
Ramos-Campoy O, Antonell A, Falgàs N et al (2020) Screening of dementia genes by whole-exome sequencing in Spanish patients with early-onset dementia: likely pathogenic, uncertain significance and risk variants. Neurobiol Aging 93:e1–e9
De Roeck A, Van den Bossche T, van der Zee J et al (2017) Deleterious ABCA7 mutations and transcript rescue mechanisms in early onset Alzheimerʼs disease. Acta Neuropathol 134:475–487
Teunissen CE, Petzold A, Bennett JL et al (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73:1914–1922
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Jack CR Jr, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimerʼs disease. Alzheimers Dement 14:535–562
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimerʼs disease at 25 years. EMBO Mol Med 8:595–608
Karran E, De Strooper B (2022) The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov 21:306–318
Li J, Xu M, Zhou H, Ma J, Potter H (1997) Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell 90:917–927
De Strooper B, Beullens M, Contreras B et al (1997) Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J Biol Chem 272:3590–3598
Li X, Dang S, Yan C, Gong X, Wang J, Shi Y (2013) Structure of a presenilin family intramembrane aspartate protease. Nature 493:56–61
Annaert WG, Levesque L, Craessaerts K et al (1999) Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol 147:277–294
Henricson A, Käll L, Sonnhammer EL (2005) A novel transmembrane topology of presenilin based on reconciling experimental and computational evidence. Febs j 272:2727–2733
Laudon H, Hansson EM, Melén K et al (2005) A nine-transmembrane domain topology for presenilin 1. J Biol Chem 280:35352–35360
Citron M, Westaway D, Xia W et al (1997) Mutant presenilins of Alzheimerʼs disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med 3:67–72
Wolozin B, Iwasaki K, Vito P et al (1996) Participation of presenilin 2 in apoptosis: enhanced basal activity conferred by an Alzheimer mutation. Science 274:1710–1713
Alves da Costa C, Paitel E, Mattson MP et al (2002) Wild-type and mutated presenilins 2 trigger p53-dependent apoptosis and down-regulate presenilin 1 expression in HEK293 human cells and in murine neurons. Proc Natl Acad Sci U S A 99:4043–4048
Araki W, Yuasa K, Takeda S et al (2001) Pro-apoptotic effect of presenilin 2 (PS2) overexpression is associated with down-regulation of Bcl-2 in cultured neurons. J Neurochem 79:1161–1168
Youn YC, Bagyinszky E, Kim H, Choi BO, An SS, Kim S (2014) Probable novel PSEN2 Val214Leu mutation in Alzheimer’s disease supported by structural prediction. BMC Neurol 14:105
An SS, Park SA, Bagyinszky E et al (2016) A genetic screen of the mutations in the Korean patients with early-onset Alzheimerʼs disease. Clin Interv Aging 11:1817–1822
Shi Z, Wang Y, Liu S et al (2015) Clinical and neuroimaging characterization of Chinese dementia patients with PSEN1 and PSEN2 mutations. Dement Geriatr Cogn Disord 39:32–40
Li D, Parks SB, Kushner JD et al (2006) Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet 79:1030–1039
Kaminski WE, Orsó E, Diederich W, Klucken J, Drobnik W, Schmitz G (2000) Identification of a novel human sterol-sensitive ATP-binding cassette transporter (ABCA7). Biochem Biophys Res Commun 273:532–538
Bossaerts L, van de HendrickxCraen E, Cacace R, Asselbergh B, Van Broeckhoven C (2022) Rare missense mutations in ABCA7 might increase Alzheimer’s disease risk by plasma membrane exclusion. Acta Neuropathol Commun 10:43
Tanaka N, Abe-Dohmae S, Iwamoto N, Yokoyama S (2011) Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J Atheroscler Thromb 18:274–281
Sakae N, Liu CC, Shinohara M et al (2016) ABCA7 deficiency accelerates amyloid-β generation and Alzheimerʼs neuronal pathology. J Neurosci 36:3848–3859
Fu Y, Hsiao JH, Paxinos G, Halliday GM, Kim WS (2016) ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J Alzheimers Dis 54:569–584
Allen M, Lincoln SJ, Corda M et al (2017) ABCA7 loss-of-function variants, expression, and neurologic disease risk. Neurology Genetics 3:e126
Le Guennec K, Nicolas G, Quenez O et al (2016) ABCA7 rare variants and Alzheimer disease risk. Neurology 86:2134–2137
Van den Bossche T, Sleegers K, Cuyvers E et al (2016) Phenotypic characteristics of Alzheimer patients carrying an ABCA7 mutation. Neurology 86:2126–2133
Campbell AS, Ho CCG, Atık M et al (2022) Clinical Deep Phenotyping of ABCA7 Mutation Carriers. Neurol Genet 8:e655
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ (2020) Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol 16:30–42
Switzer AR, Cheema I, McCreary CR et al (2020) Cerebrovascular reactivity in cerebral amyloid angiopathy, Alzheimer disease, and mild cognitive impairment. Neurology 95:e1333–e1340
Weldon Furr J, Morales-Scheihing D, Manwani B, Lee J, McCullough LD (2019) Cerebral amyloid angiopathy, Alzheimerʼs disease and MicroRNA: miRNA as diagnostic biomarkers and potential therapeutic targets. NeuroMol Med 21:369–390
Caselli RJ, Reiman EM (2013) Characterizing the preclinical stages of Alzheimerʼs disease and the prospect of presymptomatic intervention. J Alzheimer’s Dis : JAD 33(Suppl 1):S405-416
Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ (2020) Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol 16:30–42
Yamada M (2015) Cerebral amyloid angiopathy: emerging concepts. J Stroke 17:17–30
Charidimou A, Boulouis G, Frosch MP et al (2022) The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 21:714–725
Sutherland HG, Albury CL, Griffiths LR (2019) Advances in genetics of migraine. J Headache Pain 20:72
Ringman JM, Romano JD, Medina LD et al (2008) Increased prevalence of significant recurrent headache in preclinical familial Alzheimerʼs disease mutation carriers. Dement Geriatr Cogn Disord 25:380–384
Author information
Authors and Affiliations
Contributions
All authors have sufficiently participated in this work and take public responsibility for its content.
Corresponding author
Ethics declarations
Ethics statement
The study involving human participants was reviewed and approved by the Ethics Committee of Xiangya Hospital of Central South University.
Consent to participate
Informed consent to participate in the study was obtained from participants included in the study.
Consent for publication
The participants have consented to the submission of their case and data to the journal.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gan, J., Zhou, H., Liu, C. et al. PSEN2 and ABCA7 variants causing early-onset preclinical pathological changes in Alzheimer's disease: a case report and literature review. Neurol Sci 44, 1987–2001 (2023). https://doi.org/10.1007/s10072-023-06602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06602-5