Abstract
Objective
We aimed to investigate levels of cytokines/chemokines and immune checkpoint molecules in patients with anti-leucine-rich glioma-inactivated 1 (LGI1) encephalitis.
Methods
The study recruited 12 patients with anti-LGI1 encephalitis and six non-inflammatory controls from the Qilu Hospital of Shandong University treated between January 2019 and December 2020. Serum levels of 30 cytokines/chemokines and 10 checkpoint molecules were measured in participants of both the groups.
Results
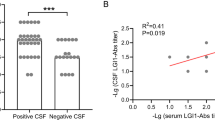
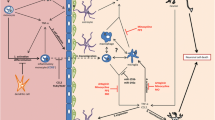
In contrast to those in the control group, 24 cytokines/chemokines and 5 immune checkpoint molecules were differentially expressed in patients with anti-LGI1 encephalitis, with 14 cytokines being upregulated and 10 being downregulated. There were 1033 enriched biological processes and 61 enriched Kyoto Encyclopedia of Genes and Genomes signaling pathways.
Conclusion
A wide range of cytokines/chemokines and immune checkpoint molecules are implicated in immune regulation in anti-LGI1 encephalitis, indicating that they may serve as important targets in the development and treatment of the disease.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Qiao S, Wu HK, Liu LL et al (2021) Characteristics and prognosis of autoimmune encephalitis in the east of china: a multi-center study. Front Neurol 12:642078. https://doi.org/10.3389/fneur.2021.642078
Graus F, Titulaer MJ, Balu R et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Titulaer MJ, Day GS (2021) Autoimmune encephalitis in first episode psychoses: all smoke and no fire? Neurology 97:16–17. https://doi.org/10.1212/WNL.0000000000012195
Qiao S, Wu HK, Liu LL et al (2021) Clinical features and long-term outcomes of anti-leucine-rich glioma-inactivated 1 encephalitis: a multi-center study. Neuropsychiatr Dis Treat 17:203–212. https://doi.org/10.2147/NDT.S292343
Ye Z, Jin Y, Xu H et al (2021) Effectiveness of immunotherapy in a CASPR2 and LGI1 antibody-positive elderly patient with Isaacs’ syndrome: a case study. Acta Neurol Belg 121:577–579. https://doi.org/10.1007/s13760-020-01446-8
Li X, Yuan J, Liu L, Hu W (2019) Antibody-LGI 1 autoimmune encephalitis manifesting as rapidly progressive dementia and hyponatremia: a case report and literature review. BMC Neurol 19:19. https://doi.org/10.1186/s12883-019-1251-4
Ciano-Petersen NL, Cabezudo-Garcia P, Muniz-Castrillo S, Honnorat J, Serrano-Castro PJ, Oliver-Martos B (2021) Current status of biomarkers in anti-n-methyl-d-aspartate receptor encephalitis. Int J Mol Sci 22.https://doi.org/10.3390/ijms222313127
Kothur K, Wienholt L, Mohammad SS et al (2016) Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, anti-NMDAR and enteroviral encephalitis. PLoS One 11:e0161656. https://doi.org/10.1371/journal.pone.0161656
Fominykh V, Brylev L, Gaskin V et al (2019) Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab Brain Dis 34:1473–1485. https://doi.org/10.1007/s11011-019-00452-x
Zou C, Pei S, Yan W et al (2020) Cerebrospinal fluid osteopontin and inflammation-associated cytokines in patients with anti-N-Methyl-D-aspartate receptor encephalitis. Front Neurol 11:519692. https://doi.org/10.3389/fneur.2020.519692
Leypoldt F, Hoftberger R, Titulaer MJ et al (2015) Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol 72:180–186. https://doi.org/10.1001/jamaneurol.2014.2956
Lin YT, Yang X, Lv JW, Liu XW, Wang SJ (2019) CXCL13 is a biomarker of anti-leucine-rich glioma-inactivated protein 1 encephalitis patients. Neuropsychiatr Dis Treat 15:2909–2915. https://doi.org/10.2147/NDT.S222258
Velasco R, Villagran M, Jove M et al (2021) Encephalitis induced by immune checkpoint inhibitors: a systematic review. JAMA Neurol 78:864–873. https://doi.org/10.1001/jamaneurol.2021.0249
Vogrig A, Muniz-Castrillo S, Joubert B et al (2021) Cranial nerve disorders associated with immune checkpoint inhibitors. Neurology 96:e866–e875. https://doi.org/10.1212/WNL.0000000000011340
Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R (2019) PD-1/PD-L1 immune checkpoint: potential target for cancer therapy. J Cell Physiol 234:1313–1325. https://doi.org/10.1002/jcp.27172
Chakrabarti R, Kapse B, Mukherjee G (2019) Soluble immune checkpoint molecules: serum markers for cancer diagnosis and prognosis. Cancer Rep 2:e1160. https://doi.org/10.1002/cnr2.1160
Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17:49–59. https://doi.org/10.1038/nri.2016.123
Kothur K, Wienholt L, Brilot F, Dale RC (2016) CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 77:227–237. https://doi.org/10.1016/j.cyto.2015.10.001
Nicolás LC, Sergio MC, Cristina B et al (2022) Cytokine dynamics and targeted immunotherapies in autoimmune encephalitis. Brain Commun 4:fcac196. https://doi.org/10.1093/braincomms/fcac196
Péter K, Alexander G, Karina G et al (2020) Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis. Cytokine 135:155226. https://doi.org/10.1016/j.cyto.2020.155226
Canan U, Erdem T, Murat K et al (2012) Comparison of the cytokine profiles of patients with neuronal-antibody-associated central nervous system disorders. Int J Neurosci 122:284–289. https://doi.org/10.3109/00207454.2011
Griffith SP, Malpas CB, Alpitsis R, O’Brien TJ, Monif M (2020) The neuropsychological spectrum of anti-LGI1 antibody mediated autoimmune encephalitis. J Neuroimmunol 345:577271. https://doi.org/10.1016/j.jneuroim.2020.577271
Uzawa A, Mori M, Arai K et al (2010) Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler 16:1443–1452. https://doi.org/10.1177/1352458510379247
Matsushita T, Tateishi T, Isobe N et al (2013) Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS One 8:e61835. https://doi.org/10.1371/journal.pone.0061835
Wang H, Wang K, Zhong X et al (2012) Cerebrospinal fluid BAFF and APRIL levels in neuromyelitis optica and multiple sclerosis patients during relapse. J Clin Immunol 32:1007–1011. https://doi.org/10.1007/s10875-012-9709-9
Alvarez E, Piccio L, Mikesell RJ et al (2013) CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler 19:1204–1208. https://doi.org/10.1177/1352458512473362
Liu J, Liu L, Kang W et al (2020) Cytokines/chemokines: potential biomarkers for non-paraneoplastic anti-n-methyl-d-aspartate receptor encephalitis. Front Neurol 11:582296. https://doi.org/10.3389/fneur.2020.582296
Deng B, Liu XN, Li X, Zhang X, Quan C, Chen XJ (2017) Raised cerebrospinal fluid BAFF and APRIL levels in anti-N-methyl-d-aspartate receptor encephalitis: correlation with clinical outcome. J Neuroimmunol 305:84–91. https://doi.org/10.1016/j.jneuroim.2017.01.012
Liba Z, Kayserova J, Elisak M et al (2016) Anti-N-methyl-D-aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J Neuroinflammation 13:55. https://doi.org/10.1186/s12974-016-0507-9
Zhu J, Li Y, Zheng D et al (2019) Elevated serum and cerebrospinal fluid CD138 in patients with anti-n-methyl-d-aspartate receptor encephalitis. Front Mol Neurosci 12:116. https://doi.org/10.3389/fnmol.2019.00116
Levraut M, Bourg V, Capet N et al (2021) Cerebrospinal fluid IL-17A could predict acute disease severity in non-NMDA-receptor autoimmune encephalitis. Front Immunol 12:673021. https://doi.org/10.3389/fimmu.2021.673021
Orabona C, Mondanelli G, Puccetti P, Grohmann U (2018) Immune checkpoint molecules, personalized immunotherapy, and autoimmune diabetes. Trends Mol Med 24:931–941. https://doi.org/10.1016/j.molmed.2018.08.005
Xu Y, Fu Y, Zhu B, Wang J, Zhang B (2020) Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front Immunol 11:2023. https://doi.org/10.3389/fimmu.2020.02023
Yordduangjun N, Dishion E, McKnight CA, Caplan JP (2021) Immune checkpoint inhibitor-associated autoimmune encephalitis. J Acad Consult Liaison Psychiatry 62:115–118. https://doi.org/10.1016/j.psym.2020.08.011
Nalbantoglu M, Altunrende B, Tuncer OG, Akman G (2021) Autoimmune encephalitis after treatment of Hodgkin’s lymphoma with the immune checkpoint inhibitor nivolumab. Noro Psikiyatr Ars 58:163–165. https://doi.org/10.29399/npa.23353
Williams TJ, Benavides DR, Patrice KA et al (2016) Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 73:928–933. https://doi.org/10.1001/jamaneurol.2016.1399
Schneider S, Potthast S, Komminoth P, Schwegler G, Bohm S (2017) PD-1 checkpoint inhibitor associated autoimmune encephalitis. Case Rep Oncol 10:473–478. https://doi.org/10.1159/000477162
Sakuishi K, Ngiow SF, Sullivan JM et al (2013) TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2:e23849. https://doi.org/10.4161/onci.23849
Ralser DJ, Klumper N, Gevensleben H et al (2021) Molecular and immune correlates of PDCD1 (PD-1), PD-L1 (CD274), and PD-L2 (PDCD1LG2) DNA methylation in triple negative breast cancer. J Immunother 44:319–324. https://doi.org/10.1097/CJI.0000000000000384
Hellbacher E, Sundstrom C, Molin D, Baecklund E, Hollander P (2022) Expression of PD-1, PD-L1 and PD-L2 in lymphomas in patients with pre-existing rheumatic diseases-a possible association with high rheumatoid arthritis disease activity. Cancers (Basel) 14.https://doi.org/10.3390/cancers14061509
Rieder SA, Wang J, White N et al (2021) B7–H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol 18:1503–1511. https://doi.org/10.1038/s41423-020-0361-7
Nunes JA, Olive D (2022) CD28 costimulation promotes an antitumor CD8(+) T cell response in myeloid antigen-presenting cell niches. Cell Mol Immunol 19:147–149. https://doi.org/10.1038/s41423-021-00818-1
Estrada-Capetillo L, Aragoneses-Fenoll L, Dominguez-Soto A et al (2021) CD28 is expressed by macrophages with anti-inflammatory potential and limits their T-cell activating capacity. Eur J Immunol 51:824–834. https://doi.org/10.1002/eji.202048806
Acknowledgements
The authors thank all participants and their families for their cooperation.
Funding
This work was supported by the National Natural Science Foundation (Grant number 81873786).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of the Qilu Hospital of Shandong University (No. KYLL-202008–044). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, S., Zhang, Sc., Li, Hy. et al. Cytokines/chemokines and immune checkpoint molecules in anti-leucine-rich glioma-inactivated 1 encephalitis. Neurol Sci 44, 1017–1029 (2023). https://doi.org/10.1007/s10072-022-06526-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06526-6