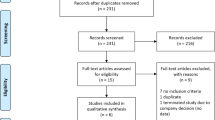
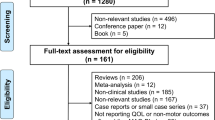
Abstract
Background
Rotigotine transdermal patch (TP) is a useful dopaminergic medication for Parkinson’s disease (PD). This meta-analysis attempted to evaluate the effects of rotigotine TP on motor performance, activities of daily living (ADL) limitations, and sleep disturbances in patients with PD.
Methods
Only randomized controlled clinical trials (RCTs) with placebo design were included in this study. The clinical outcomes, evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS III), UPDRS-II, UPDRS Part II + III, Parkinson’s Disease Sleep Scale (PDSS)-2, and adverse events (AEs) were evaluated. The Jadad scale was used to evaluate study quality.
Results
A total of 16 RCTs with 4682 patients with PD were enrolled in this study. We found that rotigotine TP significantly reduced the UPDRS-III, UPDRS-II, and UPDRS Part II + III scores, indicating that rotigotine TP led to a significant amelioration of movement symptoms and ADL limitations. Moreover, we found that rotigotine TP significantly reduced PDSS-2 scores, suggesting that rotigotine TP led to a remarkable improvement in sleep quality. Meanwhile, compared with the placebo group, patients taking rotigotine TP did not have added incidence of AEs.
Conclusion
This study verified the efficacy and safety of rotigotine TP in treating PD. The findings of the present study provide compelling evidence concerning and insight into clinical usage of rotigotine TP. Future studies will focus on more non-motor symptoms affected by rotigotine TP.




Similar content being viewed by others
References
Asakawa T, Sugiyama K, Nozaki T, Sameshima T, Kobayashi S, Wang L, Hong Z, Chen S, Li C, Namba H (2019) Can the latest computerized technologies revolutionize conventional assessment tools and therapies for a neurological disease? The example of Parkinson’s disease. Neurol Med Chir (Tokyo) 59(3):69–78. https://doi.org/10.2176/nmc.ra.2018-0045
Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha JF, McCrory M, Soares-da-Silva P, Investigators B-S (2017) Opicapone as adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 74(2):197–206. https://doi.org/10.1001/jamaneurol.2016.4703
Goudreau JL, Pérez A, Aminoff MJ, Boyd JT, Burau KD, Christine CW, Leehey M, Morgan JC, Investigators N-P (2016) Choice of dopaminergic therapy among early, mild Parkinson disease subjects in North America. J Neurol Sci 366:74–81
LeWitt PA, Hauser RA, Grosset DG, Stocchi F, Saint-Hilaire MH, Ellenbogen A, Leinonen M, Hampson NB, DeFeo-Fraulini T, Freed MI, Kieburtz KD (2016) A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.26611
Caraco Y, Oren S, Yacoby-Zeevi O (2013) ND0612, a novel formulation of levodopa/carbidopa for continuous, subcutaneous administration, achieves steady-state levodopa plasma concentration in Parkinson’s disease patients. Mov Disord 79:56
Giladi N, Caraco Y, Gurevitch T, Djaldetti R, Cohen Y, Yacobi-Zeevi O, Oren S (2015) Pharmacokinetics and safety of ND0612L (levodopa/carbidopa for subcutaneous infusion): results from a phase II study in moderate to severe Parkinson’s disease. Age (years) 63(7.4):64–65
Chang FC, Kwan V, van der Poorten D, Mahant N, Wolfe N, Ha AD, Griffith JM, Tsui D, Kim SD, Fung VS (2016) Intraduodenal levodopa-carbidopa intestinal gel infusion improves both motor performance and quality of life in advanced Parkinson’s disease. J Clin Neurosci 25:41–45
Pellicano C, Benincasa D, Fanciulli A, Latino P, Giovannelli M, Pontieri FE (2013) The impact of extended release dopamine agonists on prescribing patterns for therapy of early Parkinson’s disease: an observational study. Eur J Med Res 18(1):1
Md S, Karim S, Saker SR, Gie OA, Hooi LC, Yee PH, Kang AWC, Zhe CK, Ian N, Aldawsari HM, Hosny KM, Alhakamy NA (2020) Current status and challenges in rotigotine delivery. Curr Pharm Des 26(19):2222–2232. https://doi.org/10.2174/1381612826666200316154300
Nomoto M, Iwaki H, Kondo H, Sakurai M (2018) Efficacy and safety of rotigotine in elderly patients with Parkinson’s disease in comparison with the non-elderly: a post hoc analysis of randomized, double-blind, placebo-controlled trials. J Neurol 265(2):253–265. https://doi.org/10.1007/s00415-017-8671-0
Frampton JE (2019) Rotigotine transdermal patch: a review in Parkinson’s disease. CNS Drugs 33(7):707–718. https://doi.org/10.1007/s40263-019-00646-y
Baldwin CM, Keating GM (2007) Rotigotine transdermal patch: a review of its use in the management of Parkinson’s disease. CNS Drugs 21(12):1039–1055. https://doi.org/10.2165/00023210-200721120-00007
Sanford M, Scott LJ (2011) Rotigotine transdermal patch: a review of its use in the treatment of Parkinson’s disease. CNS Drugs 25(8):699–719. https://doi.org/10.2165/11206750-000000000-00000
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Zhou CQ, Li SS, Chen ZM, Li FQ, Lei P, Peng GG (2013) Rotigotine transdermal patch in Parkinson’s disease: a systematic review and meta-analysis. PLoS ONE 8(7):e69738. https://doi.org/10.1371/journal.pone.0069738
Chen F, Jin L, Nie Z (2017) Safety and efficacy of rotigotine for treating Parkinson’s disease: a meta-analysis of randomised controlled trials. J Pharm Pharm Sci 20 (0):285–294. https://doi.org/10.18433/J3Q35D
Asakawa T, Fang H, Sugiyama K, Nozaki T, Kobayashi S, Hong Z, Suzuki K, Mori N, Yang Y, Hua F, Ding G, Wen G, Namba H, Xia Y (2016) Human behavioral assessments in current research of Parkinson’s disease. Neurosci Biobehav Rev 68:741–772. https://doi.org/10.1016/j.neubiorev.2016.06.036
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12. https://doi.org/10.1016/0197-2456(95)00134-4
Jiang F, Yang T, Yin H, Guo Y, Namba H, Sun Z, Asakawa T (2018) Evidence for the use of acupuncture in treating Parkinson’s disease: update of information from the past 5 years, a mini review of the literature. Front Neurol 9:596. https://doi.org/10.3389/fneur.2018.00596
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 343:d5928. https://doi.org/10.1136/bmj.d5928
Bhidayasiri R, Sringean J, Chaiwong S, Anan C, Penkeaw N, Leaknok A, Boonpang K, Saksornchai K, Rattanachaisit W, Thanawattano C, Jagota P (2017) Rotigotine for nocturnal hypokinesia in Parkinson’s disease: quantitative analysis of efficacy from a randomized, placebo-controlled trial using an axial inertial sensor. Parkinsonism Relat Disord 44:124–128. https://doi.org/10.1016/j.parkreldis.2017.08.010
Pierantozzi M, Placidi F, Liguori C, Albanese M, Imbriani P, Marciani MG, Mercuri NB, Stanzione P, Stefani A (2016) Rotigotine may improve sleep architecture in Parkinson’s disease: a double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med 21:140–144. https://doi.org/10.1016/j.sleep.2016.01.016
Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, Boroojerdi B, Investigators SP (2007) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6(6):513–520. https://doi.org/10.1016/S1474-4422(07)70108-4
Giladi N, Boroojerdi B, Korczyn AD, Burn DJ, Clarke CE, Schapira AH, investigators SP, (2007) Rotigotine transdermal patch in early Parkinson’s disease: a randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 22(16):2398–2404. https://doi.org/10.1002/mds.21741
Mizuno Y, Nomoto M, Hasegawa K, Hattori N, Kondo T, Murata M, Takeuchi M, Takahashi M, Tomida T, Rotigotine Trial G (2014) Rotigotine vs ropinirole in advanced stage Parkinson’s disease: a double-blind study. Parkinsonism Relat Disord 20(12):1388–1393. https://doi.org/10.1016/j.parkreldis.2014.10.005
Hauser RA, Slawek J, Barone P, Dohin E, Surmann E, Asgharnejad M, Bauer L (2016) Evaluation of rotigotine transdermal patch for the treatment of apathy and motor symptoms in Parkinson’s disease. BMC Neurol 16:90. https://doi.org/10.1186/s12883-016-0610-7
Nicholas AP, Borgohain R, Chana P, Surmann E, Thompson EL, Bauer L, Whitesides J, Elmer LW, Investigators SPS (2014) A randomized study of rotigotine dose response on “off” time in advanced Parkinson’s disease. J Parkinsons Dis 4(3):361–373. https://doi.org/10.3233/JPD-130320
LeWitt PA, Lyons KE, Pahwa R, Group SPS (2007) Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology 68(16):1262–1267. https://doi.org/10.1212/01.wnl.0000259516.61938.bb
Parkinson Study G (2003) A controlled trial of rotigotine monotherapy in early Parkinson’s disease. Arch Neurol 60(12):1721–1728. https://doi.org/10.1001/archneur.60.12.1721
Chung SJ, Asgharnejad M, Bauer L, Ramirez F, Jeon B (2016) Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother 17(11):1453–1461. https://doi.org/10.1080/14656566.2016.1202917
Antonini A, Bauer L, Dohin E, Oertel WH, Rascol O, Reichmann H, Schmid M, Singh P, Tolosa E, Chaudhuri KR (2015) Effects of rotigotine transdermal patch in patients with Parkinson’s disease presenting with non-motor symptoms - results of a double-blind, randomized, placebo-controlled trial. Eur J Neurol 22(10):1400–1407. https://doi.org/10.1111/ene.12757
Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, Dioszeghy P, Hill D, Anderson T, Myllyla V, Kassubek J, Steiger M, Zucconi M, Tolosa E, Poewe W, Surmann E, Whitesides J, Boroojerdi B, Chaudhuri KR, Recover Study G (2011) Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26(1):90–99. https://doi.org/10.1002/mds.23441
Jankovic J, Watts RL, Martin W, Boroojerdi B (2007) Transdermal rotigotine: double-blind, placebo-controlled trial in Parkinson disease. Arch Neurol 64(5):676–682. https://doi.org/10.1001/archneur.64.5.676
Mizuno Y, Nomoto M, Kondo T, Hasegawa K, Murata M, Takeuchi M, Ikeda J, Tomida T, Hattori N, Rotigotine Trial G (2013) Transdermal rotigotine in early stage Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Mov Disord 28(10):1447–1450. https://doi.org/10.1002/mds.25537
Zhang ZX, Shang HF, Hu X, Chen S, Zhao Z, Du X, Surmann E, Bauer L, Asgharnejad M (2016) Rotigotine transdermal patch in Chinese patients with early Parkinson’s disease: a randomized, double-blind, placebo-controlled pivotal study. Parkinsonism Relat Disord 28:49–55. https://doi.org/10.1016/j.parkreldis.2016.04.022
Nomoto M, Mizuno Y, Kondo T, Hasegawa K, Murata M, Takeuchi M, Ikeda J, Tomida T, Hattori N (2014) Transdermal rotigotine in advanced Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. J Neurol 261(10):1887–1893. https://doi.org/10.1007/s00415-014-7427-3
Rascol O, Zesiewicz T, Chaudhuri KR, Asgharnejad M, Surmann E, Dohin E, Nilius S, Bauer L (2016) A randomized controlled exploratory pilot study to evaluate the effect of rotigotine transdermal patch on Parkinson’s disease-associated chronic pain. J Clin Pharmacol 56(7):852–861. https://doi.org/10.1002/jcph.678
Pfeiffer RF (2005) A promising new technology for Parkinson’s disease. Neurology 65(2 Suppl 1):S6-10. https://doi.org/10.1212/wnl.65.2_suppl_1.s6
Elshoff JP, Cawello W, Andreas JO, Mathy FX, Braun M (2015) An update on pharmacological, pharmacokinetic properties and drug-drug interactions of rotigotine transdermal system in Parkinson’s disease and restless legs syndrome. Drugs 75(5):487–501. https://doi.org/10.1007/s40265-015-0377-y
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67(5):589–595. https://doi.org/10.1001/archneurol.2010.65
Ondo WG, Lai D (2008) Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord 14(1):28–32. https://doi.org/10.1016/j.parkreldis.2007.05.006
Antonini A, Chaudhuri KR, Boroojerdi B, Asgharnejad M, Bauer L, Grieger F, Weintraub D (2016) Impulse control disorder related behaviours during long-term rotigotine treatment: a post hoc analysis. Eur J Neurol 23(10):1556–1565. https://doi.org/10.1111/ene.13078
Moore TJ, Glenmullen J, Mattison DR (2014) Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med 174(12):1930–1933. https://doi.org/10.1001/jamainternmed.2014.5262
Wingo TS, Evatt M, Scott B, Freeman A, Stacy M (2009) Impulse control disorders arising in 3 patients treated with rotigotine. Clin Neuropharmacol 32(2):59–62. https://doi.org/10.1097/WNF.0B013E3181684542
Schreglmann SR, Gantenbein AR, Eisele G, Baumann CR (2012) Transdermal rotigotine causes impulse control disorders in patients with restless legs syndrome. Parkinsonism Relat Disord 18(2):207–209. https://doi.org/10.1016/j.parkreldis.2011.10.010
Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, HerranzBarcenas A, Vela L, Sanchez Alonso P, Mata M, Olmedilla Gonzalez N, Mahillo Fernandez I (2014) Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry 85(8):840–844. https://doi.org/10.1136/jnnp-2013-306787
Rizos A, Sauerbier A, Antonini A, Weintraub D, Martinez-Martin P, Kessel B, Henriksen T, Falup-Pecurariu C, Silverdale M, Durner G, RokenesKarlsen K, Grilo M, Odin P, Chaudhuri KR, Europar, the IN-M-PDSG, (2016) A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur J Neurol 23(8):1255–1261. https://doi.org/10.1111/ene.13034
Asakawa T, Fang H, Sugiyama K, Nozaki T, Hong Z, Yang Y, Hua F, Ding G, Chao D, Fenoy AJ, Villarreal SJ, Onoe H, Suzuki K, Mori N, Namba H, Xia Y (2016) Animal behavioral assessments in current research of Parkinson’s disease. Neurosci Biobehav Rev 65:63–94. https://doi.org/10.1016/j.neubiorev.2016.03.016
Funding
This work was supported in part by the National Foundation of Natural Science of China (Nos. 82074539 and 81704170), Natural Science of Heilongjiang Province (No. LH2020H092), Research Fund Project for Chinese Medicine of Heilongjiang Province (No. ZHY2020-79), and Research Fund for Construction of Evidence Based Medicine for Chinese Medicine (No. 2019XZZX-ZJ005). This work was also supported by grants from the Japan Society for the Promotion of Science (Nos. 20791025, 24592157, 15k10358, and 18K08991).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
None.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, W., Wang, Q., Yang, T. et al. A meta-analysis evaluating effects of the rotigotine in Parkinson’s disease, focusing on sleep disturbances and activities of daily living. Neurol Sci 43, 5821–5837 (2022). https://doi.org/10.1007/s10072-022-06159-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06159-9