Abstract
Background
The harsh environmental conditions during space travel, particularly weightlessness, impose a major burden on the human body including the cardiovascular system. Given its importance in adjusting the cardiovascular system to environmental challenges, the autonomic nervous system has been in the focus of scientists and clinicians involved in human space flight. This review provides an overview on human autonomic research under real and simulated space conditions with a focus on orthostatic intolerance.
Methods
The authors conducted a targeted literature search using Pubmed.
Results
Overall, 120 articles were identified and included in the review.
Conclusions
Postflight orthostatic intolerance is commonly observed in astronauts and could pose major risks when landing on another celestial body. The phenomenon likely results from changes in volume status and adaptation of the autonomic nervous system to weightlessness. Over the years, various non-pharmacological and pharmacological countermeasures have been investigated. In addition to enabling safe human space flight, this research may have implications for patients with disorders affecting cardiovascular autonomic control on Earth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the first human space flight in the early 1960s of the last century by cosmonaut Yuri Gagarin, human space programs evolved rapidly. In 1969, the astronaut Neil Armstrong was the first human being to set foot on the Moon. Subsequently the American Space Shuttle program was launched, and the Russian MIR space station was established. Perhaps, the most astonishing accomplishment in recent decades was the creation of the International Space Station (ISS) [1]. The first ISS modules were launched in the late 1990s. Meanwhile, the ISS enabled the presence of human beings in low Earth orbit for more than 20 years. While ISS will orbit the Earth for a few more years, we will witness exciting new developments driven by space agencies and commercial entities. China will continue to build and utilize its recently established space station. The Artemis program, which is led by the United States National Aeronautics and Space Administration (NASA), will bring human beings back to the moon and may lay the foundation for future human missions to Mars. Finally, commercial providers have begun to offer suborbital and orbital space flights to paying customers. Thus, more and more human beings will experience space travel including individuals who would not qualify for a career as professional astronaut for medical or psychological reasons.
Given its importance in adjusting the cardiovascular system to environmental challenges, the autonomic nervous system has been in the focus of scientists and clinicians involved in human space flight. One of the highlights in this area was an international space shuttle mission dedicated to mechanistic human studies on post-space-flight orthostatic intolerance (Neurolab Mission, STS-90) [2]. The harsh environmental conditions during space travel impose a major burden on the human body including the cardiovascular system [3]. In space, even the healthiest of the healthy will experience worsening of performance and health status unless sufficient countermeasures are instituted. Among more than 30 health risks during long duration space travel, NASA scientists identified five so-called red risks based on their likelihood to occur and their implications for health and performance during and after the mission [4]. These risks comprise consequences of space radiation, isolation and confinement, a hostile and closed environment, the distance from Earth, and altered gravity. Of those, altered gravity elicits particularly strong effects on cardiovascular control through volume redistribution in the body or altered vestibular signaling among other mechanisms. However, all the other environmental challenges could also affect the autonomic nervous system. For example, altered atmosphere conditions in a closed environment could engage peripheral or central chemoreceptors which regulate the autonomic nervous system. In the event that neurons in the brain in autonomic control circuits are being hit by heavy ions, which are important and difficult to shield components of galactic cosmic radiation, grave consequences could occur [5]. Finally, the psychological stresses associated with confinement and isolation could conceivably affect the autonomic nervous system [6].
This review provides an overview on human autonomic research under real and simulated space conditions with a focus on orthostatic intolerance. In addition to enabling safe human space flight, this research may have implications for patients with disorders affecting cardiovascular autonomic control on Earth.
Orthostatic tolerance—focus on the autonomic nervous system
Environmental challenges are sensed through baroreceptors, chemoreceptors, and the vestibular system among other afferent inputs [7,8,9]. The information is integrated at the brainstem level and leads to adjustments in efferent sympathetic and parasympathetic activity. Sympathetic stimulation increases heart rate and cardiac contractility, increases vascular tone, and promotes renal sodium reabsorption through renin release and direct tubular actions. Parasympathetic activation primarily decreases heart rate at the level of the sinus node. The importance of the autonomic nervous system in cardiovascular control is illustrated by patients with severe autonomic failure. In these patients, efferent sympathetic and parasympathetic counter-regulation is almost completely lost such that hemodynamic stresses imposed by standing produce profound orthostatic hypotension [10]. Other seemingly trivial hemodynamic challenges such as eating, taking a hot shower, or drinking alcohol massively reduce blood pressure. These stresses are easily compensated for by autonomic counter-regulation in healthy young persons. However, sufficiently strong stimuli, such as exposure to hypergravity on a human centrifuge, in a high-performance aircraft, or during rocket launch or reentry may exceed the compensatory capacity of the healthy autonomic nervous system. Changes in volume status, such as acute blood loss, or cardiovascular deconditioning can also impair tolerance to gravitational challenges [11, 12]. Thus, unusually strong environmental stimuli, impaired hemodynamic reserve, or changes in autonomic nervous system control may elicit cardiovascular symptoms, particularly orthostatic intolerance and syncope [13,14,15].
The complex interactions between environment and autonomic cardiovascular control that ultimately determine orthostatic tolerance cannot be captured by single autonomic measurements. There is no gold standard approach telling the whole story. Instead, different methodologies and experimental setups have to be combined to elucidate the chain from transduction and sensing of an environmental stressor to autonomically mediated cardiovascular responses.
Orthostatic intolerance following space travel
Launch and landing of space vessels are associated with major changes in gravitational loading of the human body. During launch, engine thrust accelerates the rocket thereby imposing substantial gravitational forces on human beings. Gravitational force-induced loss of consciousness, known as g-LOC by cognoscenti, could occur. During reentry into the atmosphere and the landing phase, the spacecraft rapidly decelerates which also generates gravitational stress to the body, normally approximately 3 to 5 times the gravity of the Earth. During space shuttle reentry, heart rates exceeding 150 beats/min have been reported. The response likely resulted from combined physiological and psychological stresses [15, 16]. Thanks to gifted space engineers who incorporate human physiology knowledge in their designs, gravitational loading of the cardiovascular system is kept in a safe range except for true emergency situations. Gravitational overloading is avoided through proper positioning of astronauts orthogonally to the acting gravity main vector (chest-to-back direction) during ascent and landing and through planning of flight trajectories among other measures. Even greater challenges to cardiovascular control can occur in other settings, such as in pilots of high-performance aircrafts who are exposed to accelerations in a head-to-foot direction, and are, therefore, not the focus of this review.
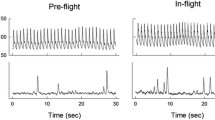
Cardiovascular autonomic symptoms related to exposure to space conditions rather than launch or landing phases have been observed early into human space flight. Physicians taking care of astronauts noted substantial orthostatic intolerance following return to Earth, a syndrome which was coined postflight orthostatic intolerance. It is safe to say that early missions were particularly heroic and stressful to astronauts. In addition to exposure to space conditions like weightlessness, astronauts were under immense psychological and physiological stress. Over the years, medical care improved together with advances in technology and individualized exercise programs. Nowadays, most astronauts are able to stand after several months onboard ISS without presyncope or syncope briefly following return to Earth. Notably, none out of 12 astronauts experienced orthostatic hypotension during the first 24 h after return from 6 months in space when investigated during activities of daily living [17]. Yet, nine out of fourteen astronauts returning from 9–14 days space shuttle missions were unable to complete a 10-min standing test conducted within 4 h after landing [15]. In the upright position, heart rate and systemic vascular resistance were markedly increased while cardiac output and stroke volume were reduced compared with measurements before the mission [15]. Russian cosmonauts returning from several months ISS missions showed a modest increase in upright heart rate within the first days after landing (Fig. 1) [18]. Upright blood pressure was well maintained. Orthostatic symptoms while generally mild-moderate and easy to manage on Earth could pose grave risks when landing on another celestial body.
Mean changes in heart rate (∆HR, top), systolic blood pressure (SBP, middle), and diastolic blood pressure (DBP, bottom) in 18 Russian cosmonauts during orthostatic testing at 60 days before space flight (pre-flight, − 60), at 30 days before space flight (− 30 pre-flight), and 3–5 days after space flight (+ 4, post-flight). p < 0.01, paired t test between pre- and post-flight values (from Tank J. et al. Clin Auton Res 2011;21(2) with permission)
Orthostatic intolerance in terrestrial models
Medical research in space is limited by the small number of potential test subjects and difficulties in conducting measurements. Assessments, which are trivial on Earth, such as obtaining and analyzing a blood sample, pose major challenges in space. Parabolic flights can produce weightlessness or reduced g-loads akin to conditions on Mars or the moon for approximately 20–30 s. We have applied the approach to assess the initial orthostatic blood pressure and heart rate responses during different gravity loads [19, 20]. Yet, the short duration of altered gravity and potential confounding through rapid changes in gravity during flight maneuvers are major limitations. Therefore, terrestrial models mimicking certain aspects of space travel have been developed and successfully applied over the years [21].
In cardiovascular autonomic research, bedrest in the head-down tilt position or dry immersion studies have been proven useful. In head-down bedrest studies, participants remain lying with the whole bed tilted head down typically by − 6°, a modest Trendelenburg position. All activities including eating and personal hygiene are conducted in this position. In dry immersion, participants are placed in a water tank with a water-resistant sheet that prevents direct contact with water. Both models unload the cardiovascular system and shift volume towards central circulatory compartments and the head. The fact that horizontal bedrest induces cardiovascular deconditioning and reduces orthostatic tolerance is known for more than 70 years [22]. One study assessed orthostatic responses before and following 6-h bedrest in a − 5° head-down position, which produces cephalad fluid shifts similarly to weightlessness, and in a + 10, + 20, or + 42° head-up position on separate days [23]. Upright heart rate was significantly increased following head-down bedrest and less so after head-up bedrest. In another study, orthostatic tolerance determined by lower body negative pressure (LBNP) decreased within 20 h of − 5° head-down bedrest [24]. The concept of applying negative pressure to the lower part of the body was originally developed for manned space flights in order to counteract the headward fluid shifts and to simulate orthostatic stress in weightlessness. In a 21-day head-down bedrest study, a duration more relevant for a space mission, orthostatic tolerance was measured by combined head-up tilt testing and LBNP and quantified as the time to abortion of the test [25]. On average, orthostatic tolerance was 21 min before and only 12 min following head-down bedrest.
Observations in space and in terrestrial models mimicking space conditions suggest that all three components affecting orthostatic tolerance may be perturbed, namely volume status, cardiovascular fitness, and autonomic nervous system control.
Changes in volume status
In the early days of human space flight, astronauts may have experienced dehydration and volume loss due to inadequate water and solute supply in the face of space motion sickness, heat stress, and excess sweating. Furthermore, astronauts avoided drinking prior to launch due to the difficulties of voiding in space [26]. However, changes in volume status and volume distribution also occur when water and nutrient supply is optimized as it is today.
Immediately when astronauts enter weightlessness, fluid and blood cells are distributed towards the head. The response is associated with paradoxical central venous pressure reduction [27, 28]. Fluid shifts towards the head and fluid redistribution from the intravascular to the interstitial and intracellular spaces contribute to face reddening and swelling—the so-called puffy face—and nose stuffiness in astronauts [29]. The internal jugular vein is dilated and shows stagnant or even retrograde flow in weightlessness [30], which may predispose to neck vein thrombosis [30, 31]. More chronically, fluid shifts towards the head may contribute to structural changes in the brain and optic disc edema, which are part of the space-flight neuro-ocular syndrome [32,33,34]. Cerebral magnetic resonance imaging of astronauts after short and long duration space missions depicted upward shift of the brain and white and gray matter volume changes [33, 35]. In another study, an increase in blood markers for brain damage emerged in the first week after return from long duration missions [36]. Mastoid effusions may also occur [37]. Invasive intracranial pressure measurements, which would be unrealistic in astronauts during space missions, have been conducted in patients with Ommaya reservoirs in hyperacute weightlessness during parabolic flights. Ommaya reservoirs are catheter systems placed in cerebral ventricles with a subcutaneous port for infusion of anticancer chemotherapy that can also be used for intracranial pressure measurements. Intracranial pressure during weightlessness differed little from supine intracranial pressure in normal gravity [38]. Possibly, intracranial pressure may not reach pathological levels in space. Yet, on Earth the brain may be protected by daily caudal volume shifts in the upright body position.
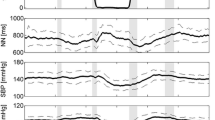
In addition to fluid shifts towards the head, plasma volume, red blood cells, and total blood volume decrease within days in space. [15] The response may be explained in part by fluid shifts from the intravascular to the intracellular compartment [39]. Furthermore, destruction of premature and young mature red blood cells may be an adaptive response to weightlessness [40]. Bioimpedance measurements suggest that there are sustained reductions in thoracic blood volume during long duration space missions (Fig. 2) [41]. Circulating mid-regional pro atrial natriuretic peptide concentrations also decrease, after a transitory 80% increase on the first day [42], in space independently of dietary sodium ingestion [41]. In a short-term − 5° head-down bedrest study, central venous pressure transiently increased followed by normalization over several hours, which differs from the response in space [24]. Yet, cephalad fluid shifts also occur and may lead to ocular changes resembling those observed in astronauts affected by the space-flight-associated neuro-ocular syndrome [43]. Mastoid effusion can also occur during head-down tilt bedrest [44]. Plasma volume decreases during head-down tilt bed rest [45]. Thus, reductions in plasma volume in weightlessness and in head-down bed rest likely put an additional strain on orthostatic tolerance.
Median thoracic fluid content (TFC) estimated by impedance measurements in 16 Russian cosmonauts before launch in the supine (SUP) and upright (UP) position, monthly onboard the International Space Station, and supine and upright after return to Earth (from Frings-Meuthen P. et al. Circulation 2020;141(19) with permission)
Cardiovascular deconditioning
Deconditioning is the expected response of the cardiovascular system to unloading be it in weightlessness or during bedrest. In astronauts, cardiopulmonary fitness was maintained after approximately 2 weeks in space but was reduced directly after return to Earth [46]. Following approximately 2-week horizontal bedrest, healthy women and men showed a marked reduction in maximal oxygen uptake [47]. It is difficult, however, to discern primary changes in cardiovascular structure and function from secondary influences of volume status and autonomic regulation on cardiopulmonary performance and orthostatic tolerance [48, 49]. Nevertheless, changes in cardiovascular structure and function may occur following space flight and in terrestrial models. In particular, reductions in left ventricular mass have been observed in astronauts following space flight and following 12 weeks horizontal bedrest [50]. However, the response of left ventricular mass to bedrest deconditioning may be heterogenous [49, 51]. One study showed reversal of cardiac atrophy within days after return from space, which may suggest that the response was mediated by fluid shifts rather than true cardiac atrophy [52]. A small heart together with reductions in volume status could contribute to orthostatic symptoms as evidenced by patients with the postural tachycardia syndrome [14, 53]. Influences of real and simulated space conditions on vascular structure and function have been recently reviewed [54]. Whether vascular changes, either on the venous or on the arterial side, contribute to orthostatic intolerance is unclear. The interpretation of the literature is complicated by the fact that in space, some aspects of cardiovascular deconditioning are addressed more than others. For example, astronauts regularly conduct endurance and strength training to maintain exercise capacity. Yet, there is no adequate substitute for terrestrial gravity challenges to the cardiovascular system.
Sympathetic nervous system adaptation
Blood pressure is maintained during orthostatic stress when sympathetic actions on heart, blood vessels, and kidney are sufficiently increased. The response requires that post-ganglionic sympathetic neurons are activated and release norepinephrine, which then engages postganglionic adrenergic receptors in cardiovascular organs or the kidney. The response is primarily terminated through neuronal re-uptake of released norepinephrine. Therefore, changes in norepinephrine release, responsiveness, or uptake could affect orthostatic tolerance following space flight.
In astronauts who underwent muscle sympathetic nerve activity measurements through microneurography, sympathetic activity was increased after space flight, both, while supine and during head-up tilt [55]. The response was proportional to reductions in cardiac stroke volume postflight. Inflight measurements of muscle sympathetic activity and norepinephrine spillover during a space shuttle mission, a remarkable accomplishment considering the complexity of these methods, showed modest increases in sympathetic neural traffic and norepinephrine release [56]. However, during parabolic flight in seated healthy men and women, muscle sympathetic nerve activity was 191% during hypergravity but only 82.8% during weightlessness compared with normal gravity [57]. In two Russian cosmonauts who underwent multiple plasma and urinary determinations before, during, and after a long-term space mission, catecholamine measurements did not show major changes in space compared to before the mission. However, these measurements increased sharply in the days following the mission [58]. An increase in plasma norepinephrine following space flight was also shown in another study [59]. Yet, eight male astronauts showed no changes in plasma noradrenaline and adrenaline concentrations during and after their long duration ISS missions with respect to preflight values, despite a 35–41% increase in cardiac output and an 8–10 mmHg decrease of arterial pressure in space [60].
Among 40 astronauts who had finished space missions of up to 16 days, those who were unable to remain standing for 10 min showed a markedly reduced increase in venous plasma norepinephrine compared to those who were able to remain standing. The group with less orthostatic tolerance also exhibited lower standing systemic vascular resistance. Women may be more likely to show such a response [61]. Astronauts with less orthostatic tolerance showed markedly raised dihydroxyphenylglycol (DHPG) plasma concentrations along with an attenuated norepinephrine response [62]. Because, norepinephrine is converted to DHPG through monoamine-oxidases following re-uptake from the synaptic cleft, the finding may indicate an increase in norepinephrine uptake and metabolism. An increase in tyramine-mediated norepinephrine release supports the idea [62]. Overall, it appears unlikely that space conditions render the sympathetic nervous system unable to respond to orthostatic stress, Yet, individual differences in sympathetic responsiveness may make an astronaut less or more likely to experience orthostatic intolerance following space flight.
Following head-down bedrest studies, participants showed unchanged or modestly increased resting sympathetic nerve traffic [63,64,65]. The sympathetic response to handgrip exercise was unchanged [65]. The reduction in blood pressure during phase 2 of the Valsalva maneuver was exacerbated by head-down bedrest; however, sympathetic activity increased appropriately [66]. A more detailed multiunit action potential analysis of sympathetic nerve recordings suggested that head-down bedrest may induce changes in neural recruitment strategies, particularly during breath holding [64]. Despite a reduction in plasma volume over time, urinary norepinephrine was substantially reduced, and plasma norepinephrine was largely unchanged towards the end of bedrest [67]. Another head-down bedrest study showed a similar response [68]. The authors reasoned that an inappropriate sympathetic response to hypovolemia could predispose to orthostatic intolerance following bedrest. The idea is supported by the observation that persons with orthostatic intolerance following head-down bedrest showed a blunted increase in sympathetic nerve traffic with standing and insufficient vasoconstriction in the splanchnic circulation and in the legs [65, 69].
Parasympathetic heart rate control
Vagal withdrawal is the first response when assuming the upright position. However, patients with a denervated heart, such as heart transplant recipients, do not show major orthostatic symptoms. Moreover, cardiac pacemakers while attenuating bradycardia during tilt table testing are of limited utility in improving orthostatic tolerance of patients with vasovagal syncope [70]. Similarly, intravenous atropine did not prevent syncope during head-up tilt testing in healthy men [71]. On the other hand, patients with sympathetic vasomotor lesions faint during orthostatic challenges despite a substantial increase in heart rate [72]. However, pharmacological norepinephrine transporter blockade, which primarily raises heart rate, prevented presyncope during head-up tilt testing in healthy persons [73]. Overall, influences of heart rate on orthostatic tolerance are limited.
Nevertheless, altered parasympathetic activity might modify orthostatic heart rate responses following space flight. Compared with before space flight, heart rate was higher, and heart rate variability was reduced on landing day [59]. Heart rate variability was well maintained in Russian cosmonauts during long duration missions. During sleep, cosmonauts onboard the MIR station showed reductions in heart rate together with an increase in heart rate variability in the high frequency range, which primarily results from parasympathetic influences on the sinus node [74]. Another study showed an opposite heart rate variability response during sleep [75]. However, maximal heart rate variability during deep breathing declined in six out of seven cosmonauts suggesting that there may be reduction in vagal reserve [76]. Following return to Earth, astronauts who were unable to complete a standing test showed an increase in heart rate variability while supine [77].
Autonomic reflex regulation
As outlined above, parasympathetic and sympathetic efferent nerves are controlled by brain stem nuclei that receive input from various afferents and higher brain areas, especially from the insula [78]. Therefore, changes in these reflex circuits could contribute to altered autonomic cardiovascular control in real or in simulated space conditions. Baroreflex and vestibular mechanisms are prime suspects. The relevance of reflexes of the low-pressure system, e.g., the heart rate response to an acute increase in blood volume (Bainbridge reflex), is unclear.
Baroreflex-mediated heart rate responses elicited through neck suction were attenuated during and after short-term space flight [79]. Another study using transfer function analysis showed reduced low-frequency gain of the baroreflex after flight [80, 81]. Repeated baroreflex sensitivity measurements using the sequence and spectral alpha techniques in four astronauts during a 16-day flight showed an initial increase in baroreflex-mediated heart rate control returning to baseline values at the end of space flight [81]. Interestingly, vagal heart rate control was reduced in 5 European but not in 5 Chinese astronauts, which raises questions regarding genetic variation in the response to space conditions [82]. In contrast, sympathetic responses to different stimuli like the Valsalva maneuver, head-up tilt, or lower body negative pressure seem to be enhanced during and after space flight [55, 56, 83]. Data about changes on autonomic reflex regulation from long-term missions is still lacking.
The central shift of body fluids after transition into weightlessness should, in theory, activate volume receptors located in central veins, pulmonary arteries, and cardiac atria with subsequent vagal withdrawal and heart rate increases [84]. However, Chinese astronauts showed heart rate reductions in space [85]. Possibly, low-pressure reflexes are masked by a dominant arterial baroreflex.
The vestibular system, which regulates autonomic efferents [86], is profoundly perturbed in weightlessness [9, 87]. Long-term space missions may attenuate vestibular influences on the sympathetic nervous system and, thereby, negatively affect orthostatic tolerance [88, 89]. Space flight may also affect the coupling between breathing and efferent autonomic activity, which is of unclear significance for orthostatic tolerance [90].
Is cerebral autoregulation perturbed after space flight?
Theoretically, an impairment in cerebral autoregulation, which maintains blood flow over a wide range in blood pressure values, could compromise orthostatic tolerance following space flight. The topic including the different methodological approaches to determine static and dynamic cerebral autoregulation has been recently reviewed [91, 92]. Remarkably, cerebral vasoconstriction and hypoperfusion with hyperacute gravity transitions during parabolic flights may predict poor orthostatic tolerance [93].
In six astronauts participating in a space shuttle mission, dynamic cerebral autoregulation was determined before, during, and after space flight at rest and during orthostatic stress. If anything, cerebral autoregulation was improved after the mission [94]. During more long-term missions, cerebral blood flow velocity increased in proportion to a reduction in hemoglobin levels [95]. The authors suggested that increased flow may have compensated reductions in blood oxygen carrying capacity. Cerebral blood flow velocities during incremental lower body negative pressure testing were lower after compared to before a head-down bedrest study [96]. While subtle changes in cerebral autoregulation may occur, particularly during more long-term missions, there is no evidence for a consistent and profound impairment.
Potential orthostatic intolerance countermeasures for space flight
Since NASA plans long duration human missions with up to 1100 days in space, development of countermeasures maintaining astronauts’ health and performance is an important goal. Given the importance of volume regulation in the pathogenesis of postflight orthostatic intolerance, interventions affecting fluid balance or sodium homeostasis appear sensible. In a bedrest study in which plasma volume was restored to pre-bedrest levels through intravenous isotonic fluids and oral salt, presyncope or syncope after bedrest was improved [97]. However, volume loading did not prevent orthostatic tachycardia. In a 7-day head-down bedrest study, treatment with the mineralocorticoid fludrocortisone over 24 h was more effective in maintaining plasma volume and orthostatic tolerance compared to oral salt and water loading [98]. Remarkably, baroreflex heart rate regulation was also better maintained in the fludrocortisone group. However, only seven persons per group were investigated. In a study conducted in space shuttle astronauts, one group received usual care, while another group increased fluid and salt intake before return to Earth. Fluid and volume loading substantially attenuated standing heart rate and stabilized blood pressure with standing [99]. Among astronauts who were treated with either a single fludrocortisone dose or placebo 7 h prior to landing, those receiving fludrocortisone did not show an obvious improvement in orthostatic tolerance [100].
Rowing exercises preserved cardiopulmonary fitness and orthostatic tolerance during a head-down tilt bedrest study [101].
In a case report, a female astronaut with post-flight orthostatic intolerance exhibited an improved orthostatic response following a subsequent space flight after ingesting a single dose of the alpha-adrenoreceptor agonist midodrine [102].
Anti-G suits as well as garment systems for the calf, thigh, and splanchnic areas were tested effective in preventing acute volume shifts and increase orthostatic tolerance after real and simulated gravity [103,104,105,106,107].
Countermeasures mimicking some or all aspects of standing on Earth while being in space have been actively investigated over many years. Thigh cuffs reducing venous drainage applied over several hours per day, an approach introduced by Russian cosmonauts [108], did not improve orthostatic tolerance following head-down bedrest [109]. However, the intervention may have had a beneficial effect on plasma volume and heart rate control. Whether lower body negative pressure training towards the end of space flight is effective in restoring orthostatic tolerance is questionable; however, cardiovascular responses to the intervention predict cardiovascular responses after returning to Earth [110]. Artificial gravity generated through a short arm centrifuge could be beneficial in attenuating the physiological deterioration in weightlessness [111]. Yet, artificial gravity alone may not suffice [49].Combinations of artificial gravity and exercise training hold promise [112]. During the Neurolab mission, four out of six crew members were exposed to 1 g on a centrifuge installed inside the Space Shuttle Columbia. After return to Earth, none of the crew members in the centrifugation group experienced orthostatic intolerance, whereas one of the two crew members in the control group showed such symptoms [113].
Applications on Earth
Observations in astronauts remind us that the ability of the autonomic nervous system to cope with terrestrial gravity cannot be taken for granted. It is no surprise that orthostatic intolerance, which comes in different expressions, is a common clinical challenge in earthlings [114, 115]. Roughly, orthostatic intolerance syndromes can be divided in neurally mediated syncope, postural tachycardia syndrome (POTS), and orthostatic hypotension. Neurally mediated syncope is among the most common reasons for emergency room visits [116]. Patients with neurally mediated syncope have perfectly normal cardiovascular autonomic control until a trigger, such as prolonged standing, sets off hypotension with or without bradycardia. Owing to its name, POTS is characterized by an excessive heart rate response and hyperadrenergic symptoms with standing [117]. POTS is among the most common autonomic nervous system disorders [118] and primarily but not exclusively affects younger women. Orthostatic hypotension, which is characterized by sustained reductions in blood pressure with standing, becomes more prevalent with increasing age [10]. Milder form of orthostatic hypotension, which is often explained by multiple factors, such as age-associated declines in autonomic regulation, deconditioning, and medications among others, is rather common in older people. Profound orthostatic hypotension usually points towards a severe underlying condition, such as multiple system atrophy, pure autonomic failure, or autoimmune autonomic ganglionopathy to name a few.
Phenotypically, orthostatic intolerance following real or simulated space flight resembles neurally mediated syncope or POTS rather than the immediate and then sustained reduction in blood pressure seen in patients with orthostatic hypotension. It appears that orthostatic intolerance following space flight is not explained by a single mechanism. Ultimately, individual predisposition, volume loss, cardiovascular deconditioning, autonomic nervous system adaptation, and, perhaps, changes in cerebral autoregulation may have an additive negative effect on orthostatic tolerance. While all the orthostatic intolerance syndromes that we encounter in patients in the clinic have different underlying causes, the observation that multiple factors determine orthostatic tolerance is clinically relevant. For example, hypovolemia makes matters worse in all these conditions. Conversely, measures attenuating venous pooling may improve neurally mediated syncope, POTS, and orthostatic hypotension. Yet, targeting a single mechanism rarely suffices in severely affected patients. For example, simply starting a patient with severe orthostatic hypotension on pressor drugs rarely controls symptoms unless other treatments, such as dietary salt intake, water ingestion, compression garments, and so on are instituted [10]. However, in severely affected patients, currently available therapies may not suffice in improving symptoms. Novel therapies such as spinal cord pacing [119] or deep brain stimulation [120] may have utility in this setting. We certainly hope that the engineering expertise, which is constantly pushed to its limit in space research, will yield new treatments for our patients with orthostatic intolerance on Earth.
References
White RJ, Averner M (2001) Humans in space. Nature 409(6823):1115–1118. https://doi.org/10.1038/35059243
Wieling W, Halliwill JR, Karemaker JM (2002) Orthostatic intolerance after space flight. J Physiol 538(Pt 1):1. https://doi.org/10.1113/jphysiol.2001.013372
Grigoriev AI, Kozlovskaya IB, Potapov AN (2002) Goals of biomedical support of a mission to Mars and possible approaches to achieving them. Aviat Space Environ Med 73(4):379–384
Patel ZS, Brunstetter TJ, Tarver WJ, Whitmire AM, Zwart SR, Smith SM, Huff JL (2020) Red risks for a journey to the red planet: the highest priority human health risks for a mission to Mars. NPJ microgravity 6(1):33. https://doi.org/10.1038/s41526-020-00124-6
Davis CM, Allen AR, Bowles DE (2021) Consequences of space radiation on the brain and cardiovascular system. J Environ Sci Health C Toxicol Carcinog 39(2):180–218. https://doi.org/10.1080/26896583.2021.1891825
Oluwafemi FA, Abdelbaki R, Lai JC, Mora-Almanza JG, Afolayan EM (2021) A review of astronaut mental health in manned missions: potential interventions for cognitive and mental health challenges. Life Sci Space Res 28:26–31. https://doi.org/10.1016/j.lssr.2020.12.002
Karemaker JM (2017) An introduction into autonomic nervous function. Physiol Meas 38(5):R89-r118. https://doi.org/10.1088/1361-6579/aa6782
Eckberg DL, Fritsch JM (1991) Human autonomic responses to actual and simulated weightlessness. J Clin Pharmacol 31(10):951–955. https://doi.org/10.1002/j.1552-4604.1991.tb03655.x
Deshpande N, Laurie SS, Lee SMC, Miller CA, Mulavara AP, Peters BT, Reschke MF, Stenger MB, Taylor LC, Wood SJ, Clément GR, Bloomberg JJ (2020) Vestibular and cardiovascular responses after long-duration spaceflight. Aerosp Med Hum Perform 91(8):621–627. https://doi.org/10.3357/amhp.5502.2020
Schroeder C, Jordan J, Kaufmann H (2013) Management of neurogenic orthostatic hypotension in patients with autonomic failure. Drugs 73(12):1267–1279. https://doi.org/10.1007/s40265-013-0097-0
Greenleaf JE, Brock PJ, Haines RF, Rositano SA, Montgomery LD, Keil LC (1977) Effect of hypovolemia, infusion, and oral rehydration on plasma electrolytes, ADH, renin activity, and +Gz tolerance. Aviat Space Environ Med 48(8):693–700
Greenleaf JE, Van Beaumont W, Bernauer EM, Haines RF, Sandler H, Sstaley RW, Yound HL, Yusken JW (1973) Effects of rehydration of +G z tolerance afterr 14-days’ bed rest. Aerosp Med 44(7):715–722
Convertino VA (2002) Mechanisms of microgravity induced orthostatic intolerance: implications for effective countermeasures. J Gravit Physiol 9(2):1–13
Levine BD, Zuckerman JH, Pawelczyk JA (1997) Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96(2):517–525. https://doi.org/10.1161/01.cir.96.2.517
Buckey JC Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG (1996) Orthostatic intolerance after spaceflight. J Appl Physiol (Bethesda, Md: 1985) 81(1):7–18. https://doi.org/10.1152/jappl.1996.81.1.7
Perez SA, Charles JB, Fortner GW, Hurst Vt, Meck JV (2003) Cardiovascular effects of anti-G suit and cooling garment during space shuttle re-entry and landing. Aviat Space Environ Med 74(7):753–757
Fu Q, Shibata S, Hastings JL, Platts SH, Hamilton DM, Bungo MW, Stenger MB, Ribeiro C, Adams-Huet B, Levine BD (2019) Impact of prolonged spaceflight on orthostatic tolerance during ambulation and blood pressure profiles in astronauts. Circulation 140(9):729–738. https://doi.org/10.1161/circulationaha.119.041050
Tank J, Baevsky RM, Funtova II, Diedrich A, Slepchenkova IN, Jordan J (2011) Orthostatic heart rate responses after prolonged space flights. Clin Auton Res 21(2):121–124. https://doi.org/10.1007/s10286-010-0106-2
Beck P, Tank J, Gauger P, Beck LEJ, Zirngibl H, Jordan J, Limper U (2018) Modeling human orthostatic responses on the Moon and on Mars. Clin Auton Res 28(3):325–332. https://doi.org/10.1007/s10286-018-0527-x
Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME (2007) Initial orthostatic hypotension: review of a forgotten condition. Clinical science (London, England: 1979) 112(3):157–165. https://doi.org/10.1042/CS20060091
Pandiarajan M, Hargens AR (2020) Ground-based analogs for human spaceflight. Front Physiol 11:716. https://doi.org/10.3389/fphys.2020.00716
Taylor HL, Henschel A et al (1949) Effects of bed rest on cardiovascular function and work performance. J Appl Physiol 2(5):223–239. https://doi.org/10.1152/jappl.1949.2.5.223
Lathers CM, Diamandis PH, Riddle JM, Mukai C, Elton KF, Bungo MW, Charles JB (1991) Orthostatic function during a stand test before and after head-up or head-down bedrest. J Clin Pharmacol 31(10):893–903. https://doi.org/10.1002/j.1552-4604.1991.tb03645.x
Gaffney FA, Nixon JV, Karlsson ES, Campbell W, Dowdey AB, Blomqvist CG (1985) Cardiovascular deconditioning produced by 20 hours of bedrest with head-down tilt (-5 degrees) in middle-aged healthy men. Am J Cardiol 56(10):634–638. https://doi.org/10.1016/0002-9149(85)91025-2
Barbic F, Heusser K, Minonzio M, Shiffer D, Cairo B, Tank J, Jordan J, Diedrich A, Gauger P, Zamuner RA, Porta A, Furlan R (2019) Effects of prolonged head-down bed rest on cardiac and vascular baroreceptor modulation and orthostatic tolerance in healthy individuals. Front Physiol 10:1061. https://doi.org/10.3389/fphys.2019.01061
Maciolek KA, Best SL (2019) How do astronauts urinate? The history of innovations enabling voiding in the void. Urology 128:8–13. https://doi.org/10.1016/j.urology.2018.11.065
Norsk P (2020) Adaptation of the cardiovascular system to weightlessness: surprises, paradoxes and implications for deep space missions. Acta Physiol (Oxf) 228(3):e13434. https://doi.org/10.1111/apha.13434
Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Blomqvist CG (1993) Central venous pressure in space. N Engl J Med 328(25):1853–1854. https://doi.org/10.1056/nejm199306243282516
Heer M, Paloski WH (2006) Space motion sickness: incidence, etiology, and countermeasures. Auton Neurosci 129(1–2):77–79. https://doi.org/10.1016/j.autneu.2006.07.014
Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB (2019) Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open 2(11):e1915011. https://doi.org/10.1001/jamanetworkopen.2019.15011
Limper U, Tank J, Ahnert T, Maegele M, Grottke O, Hein M, Jordan J (2020) The thrombotic risk of spaceflight: has a serious problem been overlooked for more than half of a century? Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa359
Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, Bloomberg JJ, Seidler RD (2019) Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol 76(4):412–419. https://doi.org/10.1001/jamaneurol.2018.4882
Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV, Zhu X, Chimowitz MI, Antonucci MU (2017) Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377(18):1746–1753. https://doi.org/10.1056/NEJMoa1705129
Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD (2011) Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118(10):2058–2069. https://doi.org/10.1016/j.ophtha.2011.06.021
Van Ombergen A, Jillings S, Jeurissen B, Tomilovskaya E, Rühl RM, Rumshiskaya A, Nosikova I, Litvinova L, Annen J, Pechenkova EV, Kozlovskaya IB, Sunaert S, Parizel PM, Sinitsyn V, Laureys S, Sijbers J, Zu Eulenburg P, Wuyts FL (2018) Brain tissue-volume changes in cosmonauts. N Engl J Med 379(17):1678–1680. https://doi.org/10.1056/NEJMc1809011
Zu Eulenburg P, Buchheim JI, Ashton NJ, Vassilieva G, Blennow K, Zetterberg H, Choukér A (2021) Changes in blood biomarkers of brain injury and degeneration following long-duration spaceflight. JAMA Neurol 78(12):1525–1527. https://doi.org/10.1001/jamaneurol.2021.3589
Inglesby DC, Antonucci MU, Spampinato MV, Collins HR, Meyer TA, Schlosser RJ, Shimada K, Roberts DR (2020) Spaceflight-associated changes in the opacification of the paranasal sinuses and mastoid air cells in astronauts. JAMA Otolaryngol Head Neck Surg 146(6):571–577. https://doi.org/10.1001/jamaoto.2020.0228
Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Levine BD (2017) Effect of gravity and microgravity on intracranial pressure. J Physiol 595(6):2115–2127. https://doi.org/10.1113/jp273557
Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM (1996) Regulation of body fluid compartments during short-term spaceflight. J Appl Physiol (Bethesda, Md: 1985) 81(1):105–116. https://doi.org/10.1152/jappl.1996.81.1.105
Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH (1996) Control of red blood cell mass in spaceflight. J Appl Physiol (Bethesda, Md: 1985) 81(1):98–104. https://doi.org/10.1152/jappl.1996.81.1.98
Frings-Meuthen P, Luchitskaya E, Jordan J, Tank J, Lichtinghagen R, Smith SM, Heer M (2020) Natriuretic peptide resetting in astronauts. Circulation 141(19):1593–1595. https://doi.org/10.1161/circulationaha.119.044203
Drummer C, Norsk P, Heer M (2001) Water and sodium balance in space. Am J Kidney Dis 38(3):684–690. https://doi.org/10.1053/ajkd.2001.27765
Laurie SS, Lee SMC, Macias BR, Patel N, Stern C, Young M, Stenger MB (2020) Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol 138(2):165–172. https://doi.org/10.1001/jamaophthalmol.2019.5261
Lecheler L, Paulke F, Sonnow L, Limper U, Schwarz D, Jansen S, Klussmann JP, Tank J, Jordan J (2021) Gravity and mastoid effusion. Am J Med 134(3):e181–e183. https://doi.org/10.1016/j.amjmed.2020.09.020
Platts SH, Martin DS, Stenger MB, Perez SA, Ribeiro LC, Summers R, Meck JV (2009) Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med 80(5 Suppl):A29-36. https://doi.org/10.3357/asem.br03.2009
Levine BD, Lane LD, Watenpaugh DE, Gaffney FA, Buckey JC, Blomqvist CG (1996) Maximal exercise performance after adaptation to microgravity. J Appl Physiol (Bethesda, Md: 1985) 81(2):686–694. https://doi.org/10.1152/jappl.1996.81.2.686
Convertino VA, Stremel RW, Bernauer EM, Greenleaf JE (1977) Cardiorespiratory responses to exercise after bed rest in men and women. Acta Astronaut 4(7–8):895–905. https://doi.org/10.1016/0094-5765(77)90020-0
Ade CJ, Broxterman RM, Barstow TJ (2015) VO(2max) and microgravity exposure: convective versus diffusive O(2) transport. Med Sci Sports Exerc 47(7):1351–1361. https://doi.org/10.1249/mss.0000000000000557
Hoffmann F, Rabineau J, Mehrkens D, Gerlach DA, Moestl S, Johannes BW, Caiani EG, Migeotte PF, Jordan J, Tank J (2021) Cardiac adaptations to 60 day head-down-tilt bed rest deconditioning. Findings from the AGBRESA study. ESC Heart Failure 8(1):729–744. https://doi.org/10.1002/ehf2.13103
Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD (2001) Cardiac atrophy after bed rest and spaceflight. J Appl Physiol (Bethesda, Md: 1985) 91(2):645–653. https://doi.org/10.1152/jappl.2001.91.2.645
Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR (2007) Cardiac atrophy in women following bed rest. J Appl Physiol (Bethesda, Md: 1985) 103(1):8–16. https://doi.org/10.1152/japplphysiol.01162.2006
Summers RL, Martin DS, Meck JV, Coleman TG (2005) Mechanism of spaceflight-induced changes in left ventricular mass. Am J Cardiol 95(9):1128–1130. https://doi.org/10.1016/j.amjcard.2005.01.033
Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD (2010) Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 55(25):2858–2868. https://doi.org/10.1016/j.jacc.2010.02.043
Hughson RL, Helm A, Durante M (2018) Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat Rev Cardiol 15(3):167–180. https://doi.org/10.1038/nrcardio.2017.157
Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG (2002) Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 538(Pt 1):331–340. https://doi.org/10.1113/jphysiol.2001.012575
Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC Jr, Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ, Robertson D (2002) Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol 538(Pt 1):321–329. https://doi.org/10.1113/jphysiol.2001.012576
Iwase S, Mano T, Cui J, Kitazawa H, Kamiya A, Miyazaki S, Sugiyama Y, Mukai C, Nagaoka S (1999) Sympathetic outflow to muscle in humans during short periods of microgravity produced by parabolic flight. Am J Physiol 277(2 Pt 2):R419-426
Kvetnansky R, Davydova NA, Noskov VB, Vigas M, Popova IA, Usakov AC, Macho L, Grigoriev AI (1988) Plasma and urine catecholamine levels in cosmonauts during long-term stay on Space Station Salyut-7. Acta Astronaut 17(2):181–186. https://doi.org/10.1016/0094-5765(88)90020-3
Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL (1994) Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol (Bethesda, Md : 1985) 77(4):1776–1783. https://doi.org/10.1152/jappl.1994.77.4.1776
Norsk P, Asmar A, Damgaard M, Christensen NJ (2015) Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol 593(3):573–584. https://doi.org/10.1113/jphysiol.2014.284869
Waters WW, Ziegler MG, Meck JV (2002) Postspaceflight orthostatic hypotension occurs mostly in women and is predicted by low vascular resistance. J Appl Physiol (Bethesda, Md: 1985) 92(2):586–594. https://doi.org/10.1152/japplphysiol.00544.2001
Meck JV, Waters WW, Ziegler MG, deBlock HF, Mills PJ, Robertson D, Huang PL (2004) Mechanisms of postspaceflight orthostatic hypotension: low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol 286(4):H1486-1495. https://doi.org/10.1152/ajpheart.00740.2003
Kamiya A, Iwase S, Michikami D, Fu Q, Mano T, Kitaichi K, Takagi K (2000) Increased vasomotor sympathetic nerve activity and decreased plasma nitric oxide release after head-down bed rest in humans: disappearance of correlation between vasoconstrictor and vasodilator. Neurosci Lett 281(1):21–24. https://doi.org/10.1016/s0304-3940(00)00804-1
Klassen SA, De Abreu S, Greaves DK, Kimmerly DS, Arbeille P, Denise P, Hughson RL, Normand H, Shoemaker JK (2018) Long-duration bed rest modifies sympathetic neural recruitment strategies in male and female participants. J Appl Physiol (Bethesda, Md: 1985) 124(3):769–779. https://doi.org/10.1152/japplphysiol.00640.2017
Shoemaker JK, Hogeman CS, Sinoway LI (1999) Contributions of MSNA and stroke volume to orthostatic intolerance following bed rest. Am J Physiol 277(4 Pt 2):R1084-1090. https://doi.org/10.1152/ajpregu.1999.277.4.r1084
Shoemaker JK, Hogeman CS, Sinoway LI (2003) Sympathetic responses to Valsalva’s manoeuvre following bed rest. Can J Appl Physiol 28(3):342–355. https://doi.org/10.1139/h03-025
Goldstein DS, Vernikos J, Holmes C, Convertino VA (1995) Catecholaminergic effects of prolonged head-down bed rest. J Appl Physiol (Bethesda, Md: 1985) 78(3):1023–1029. https://doi.org/10.1152/jappl.1995.78.3.1023
Johansen LB, Gharib C, Allevard AM, Sigaudo D, Christensen NJ, Drummer C, Norsk P (1997) Haematocrit, plasma volume and noradrenaline in humans during simulated weightlessness for 42 days. Clin Physiol (Oxford, England) 17(2):203–210. https://doi.org/10.1046/j.1365-2281.1997.02626.x
Arbeille P, Kerbeci P, Mattar L, Shoemaker JK, Hughson R (2008) Insufficient flow reduction during LBNP in both splanchnic and lower limb areas is associated with orthostatic intolerance after bedrest. Am J Physiol Heart Circ Physiol 295(5):H1846-1854. https://doi.org/10.1152/ajpheart.509.2008
el-Bedawi KM, Wahbha MA, Hainsworth R (1994) Cardiac pacing does not improve orthostatic tolerance in patients with vasovagal syncope. Clin Auton Res 4(5):233–237. https://doi.org/10.1007/bf01827427
Weissler AM, Warren JV, Estes EH Jr, McIntosh HD, Leonard JJ (1957) Vasodepressor syncope; factors influencing cardiac output. Circulation 15(6):875–882. https://doi.org/10.1161/01.cir.15.6.875
Wieling W, de Lange FJ, Jardine DL (2014) The heart cannot pump blood that it does not receive. Front Physiol 5:360. https://doi.org/10.3389/fphys.2014.00360
Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, Jordan J (2006) Norepinephrine transporter inhibition prevents tilt-induced pre-syncope. J Am Coll Cardiol 48(3):516–522. https://doi.org/10.1016/j.jacc.2006.04.073
Gundel A, Drescher J, Spatenko YA, Polyakov VV (1999) Heart period and heart period variability during sleep on the MIR space station. J Sleep Res 8(1):37–43. https://doi.org/10.1046/j.1365-2869.1999.00131.x
Xu D, Shoemaker JK, Blaber AP, Arbeille P, Fraser K, Hughson RL (2013) Reduced heart rate variability during sleep in long-duration spaceflight. Am J Physiol Regul Integr Comp Physiol 305(2):R164-170. https://doi.org/10.1152/ajpregu.00423.2012
Baevsky RM, Baranov VM, Funtova II, Diedrich A, Pashenko AV, Chernikova AG, Drescher J, Jordan J, Tank J (2007) Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J Appl Physiol (Bethesda, Md: 1985) 103(1):156–161. https://doi.org/10.1152/japplphysiol.00137.2007
Sigaudo-Roussel D, Custaud MA, Maillet A, Güell A, Kaspranski R, Hughson RL, Gharib C, Fortrat JO (2002) Heart rate variability after prolonged spaceflights. Eur J Appl Physiol 86(3):258–265. https://doi.org/10.1007/s00421-001-0551-7
Chouchou F, Mauguière F, Vallayer O, Catenoix H, Isnard J, Montavont A, Jung J, Pichot V, Rheims S, Mazzola L (2019) How the insula speaks to the heart: cardiac responses to insular stimulation in humans. Hum Brain Mapp 40(9):2611–2622. https://doi.org/10.1002/hbm.24548
Eckberg DL, Halliwill JR, Beightol LA, Brown TE, Taylor JA, Goble R (2010) Human vagal baroreflex mechanisms in space. J Physiol 588(Pt 7):1129–1138. https://doi.org/10.1113/jphysiol.2009.186650
Gisolf J, Immink RV, van Lieshout JJ, Stok WJ, Karemaker JM (2005) Orthostatic blood pressure control before and after spaceflight, determined by time-domain baroreflex method. J Appl Physiol (Bethesda, Md: 1985) 98(5):1682–1690. https://doi.org/10.1152/japplphysiol.01219.2004
Di Rienzo M, Castiglioni P, Iellamo F, Volterrani M, Pagani M, Mancia G, Karemaker JM, Parati G (2008) Dynamic adaptation of cardiac baroreflex sensitivity to prolonged exposure to microgravity: data from a 16-day spaceflight. J Appl Physiol (Bethesda, Md: 1985) 105(5):1569–1575. https://doi.org/10.1152/japplphysiol.90625.2008
Liu J, Li Y, Verheyden B, Chen S, Chen Z, Gai Y, Liu J, Gao J, Xie Q, Yuan M, Li Q, Li L, Aubert AE (2015) Is autonomic modulation different between European and Chinese astronauts? PLoS One 10(3):e0120920. https://doi.org/10.1371/journal.pone.0120920
Cox JF, Tahvanainen KU, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC Jr, Baisch FJ, Blomqvist CG, Robertson D, Eckberg DL (2002) Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. J Physiol 538(Pt 1):309–320. https://doi.org/10.1113/jphysiol.2001.012574
Crystal GJ, Salem MR (2012) The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 114(3):520–532. https://doi.org/10.1213/ANE.0b013e3182312e21
Liu Z, Wan Y, Zhang L, Tian Y, Lv K, Li Y, Wang C, Chen X, Chen S, Guo J (2015) Alterations in the heart rate and activity rhythms of three orbital astronauts on a space mission. Life Sci Space Res 4:62–66. https://doi.org/10.1016/j.lssr.2015.01.001
Yates BJ, Bolton PS, Macefield VG (2014) Vestibulo-sympathetic responses. Compr Physiol 4(2):851–887. https://doi.org/10.1002/cphy.c130041
Reschke MF, Good EF, Clément GR (2017) Neurovestibular symptoms in astronauts immediately after space shuttle and international space station missions. OTO Open 1(4):2473974x17738767. https://doi.org/10.1177/2473974x17738767
Hallgren E, Migeotte PF, Kornilova L, Delière Q, Fransen E, Glukhikh D, Moore ST, Clément G, Diedrich A, MacDougall H, Wuyts FL (2015) Dysfunctional vestibular system causes a blood pressure drop in astronauts returning from space. Sci Rep 5:17627. https://doi.org/10.1038/srep17627
Morita H, Abe C, Tanaka K (2016) Long-term exposure to microgravity impairs vestibulo-cardiovascular reflex. Sci Rep 6:33405. https://doi.org/10.1038/srep33405
Eckberg DL, Diedrich A, Cooke WH, Biaggioni I, Buckey JC Jr, Pawelczyk JA, Ertl AC, Cox JF, Kuusela TA, Tahvanainen KU, Mano T, Iwase S, Baisch FJ, Levine BD, Adams-Huet B, Robertson D, Blomqvist CG (2016) Respiratory modulation of human autonomic function: long-term neuroplasticity in space. J Physiol 594(19):5629–5646. https://doi.org/10.1113/jp271656
Olsen MH, Riberholt CG, Mehlsen J, Berg RM, Møller K (2021) Reliability and validity of the mean flow index (Mx) for assessing cerebral autoregulation in humans: a systematic review of the methodology. J Cereb Blood Flow Metab: 271678x211052588. https://doi.org/10.1177/0271678x211052588
Simpson DM, Payne SJ, Panerai RB (2021) The INfoMATAS project: methods for assessing cerebral autoregulation in stroke. J Cereb Blood Flow Metab: 271678x211029049. https://doi.org/10.1177/0271678x211029049
Serrador JM, Shoemaker JK, Brown TE, Kassam MS, Bondar RL, Schlegel TT (2000) Cerebral vasoconstriction precedes orthostatic intolerance after parabolic flight. Brain Res Bull 53(1):113–120
Iwasaki K, Levine BD, Zhang R, Zuckerman JH, Pawelczyk JA, Diedrich A, Ertl AC, Cox JF, Cooke WH, Giller CA, Ray CA, Lane LD, Buckey JC Jr, Baisch FJ, Eckberg DL, Robertson D, Biaggioni I, Blomqvist CG (2007) Human cerebral autoregulation before, during and after spaceflight. J Physiol 579(Pt 3):799–810. https://doi.org/10.1113/jphysiol.2006.119636
Iwasaki KI, Ogawa Y, Kurazumi T, Imaduddin SM, Mukai C, Furukawa S, Yanagida R, Kato T, Konishi T, Shinojima A, Levine BD, Heldt T (2021) Long-duration spaceflight alters estimated intracranial pressure and cerebral blood velocity. J Physiol 599(4):1067–1081. https://doi.org/10.1113/jp280318
Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD (1997) Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol (Bethesda, Md: 1985) 83(6):2139–2145. https://doi.org/10.1152/jappl.1997.83.6.2139
Waters WW, Platts SH, Mitchell BM, Whitson PA, Meck JV (2005) Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. Am J Physiol Heart Circ Physiol 288(2):H839-847. https://doi.org/10.1152/ajpheart.00220.2004
Vernikos J, Convertino VA (1994) Advantages and disadvantages of fludrocortisone or saline load in preventing post-spaceflight orthostatic hypotension. Acta Astronaut 33:259–266. https://doi.org/10.1016/0094-5765(94)90133-3
Bungo MW, Charles JB, Johnson PC Jr (1985) Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med 56(10):985–990
Shi SJ, South DA, Meck JV (2004) Fludrocortisone does not prevent orthostatic hypotension in astronauts after spaceflight. Aviat Space Environ Med 75(3):235–239
Hastings JL, Krainski F, Snell PG, Pacini EL, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD (2012) Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol (Bethesda, Md: 1985) 112(10):1735–1743. https://doi.org/10.1152/japplphysiol.00019.2012
Platts SH, Ziegler MG, Waters WW, Mitchell BM, Meck JV (2004) Midodrine prescribed to improve recurrent post-spaceflight orthostatic hypotension. Aviat Space Environ Med 75(6):554–556
Platts SH, Tuxhorn JA, Ribeiro LC, Stenger MB, Lee SM, Meck JV (2009) Compression garments as countermeasures to orthostatic intolerance. Aviat Space Environ Med 80(5):437–442. https://doi.org/10.3357/asem.2473.2009
Stenger MB, Brown AK, Lee SM, Locke JP, Platts SH (2010) Gradient compression garments as a countermeasure to post-spaceflight orthostatic intolerance. Aviat Space Environ Med 81(9):883–887. https://doi.org/10.3357/asem.2781.2010
Stenger MB, Lee SM, Ribeiro LC, Phillips TR, Ploutz-Snyder RJ, Willig MC, Westby CM, Platts SH (2014) Gradient compression garments protect against orthostatic intolerance during recovery from bed rest. Eur J Appl Physiol 114(3):597–608. https://doi.org/10.1007/s00421-013-2787-4
Stenger MB, Lee SM, Westby CM, Ribeiro LC, Phillips TR, Martin DS, Platts SH (2013) Abdomen-high elastic gradient compression garments during post-spaceflight stand tests. Aviat Space Environ Med 84(5):459–466. https://doi.org/10.3357/asem.3528.2013
Vil-Viliams IF, Kotovskaya AR, Lukjanuk V, Gavrilova LN, Chjuk MI, Nikolashin GF, Yarov AS, Krjutchenko SG, Klejev VV (1996) Study of the effectiveness of the “Centaur” anti-G suit [correction of suite] during exposure to +Gz accelerations after immersion. J Gravit Physiol 3(2):24–25
Arbeille P, Fomina G, Achaibou F, Pottier J, Kotovskaya A (1995) Cardiac and vascular adaptation to 0g with and without thigh cuffs (Antares 14 and Altair 21 day Mir spaceflights). Acta Astronaut 36(8–12):753–762. https://doi.org/10.1016/0094-5765(95)00166-2
Custaud MA, Millet C, Frutoso J, Maillet A, Gauquelin G, Gharib C, Fortrat JO (2000) No effect of venoconstrictive thigh cuffs on orthostatic hypotension induced by head-down bed rest. Acta Physiol Scand 170(2):77–85. https://doi.org/10.1046/j.1365-201x.2000.00763.x
Charles JB, Lathers CM (1994) Summary of lower body negative pressure experiments during space flight. J Clin Pharmacol 34(6):571–583. https://doi.org/10.1002/j.1552-4604.1994.tb02009.x
Clément GR, Bukley AP, Paloski WH (2015) Artificial gravity as a countermeasure for mitigating physiological deconditioning during long-duration space missions. Front Syst Neurosci 9:92. https://doi.org/10.3389/fnsys.2015.00092
Frett T, Green DA, Arz M, Noppe A, Petrat G, Kramer A, Kuemmel J, Tegtbur U, Jordan J (2020) Motion sickness symptoms during jumping exercise on a short-arm centrifuge. PLoS One 15(6):e0234361. https://doi.org/10.1371/journal.pone.0234361
Moore ST, Diedrich A, Biaggioni I, Kaufmann H, Raphan T, Cohen B (2005) Artificial gravity: a possible countermeasure for post-flight orthostatic intolerance. Acta Astronaut 56(9–12):867–876. https://doi.org/10.1016/j.actaastro.2005.01.012
Low PA (2015) Neurogenic orthostatic hypotension: pathophysiology and diagnosis. Am J Manag Care 21(13 Suppl):s248-257
Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG (2011) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21(2):69–72. https://doi.org/10.1007/s10286-011-0119-5
Mathias CJ, Deguchi K, Schatz I (2001) Observations on recurrent syncope and presyncope in 641 patients. Lancet (London, England) 357(9253):348–353. https://doi.org/10.1016/s0140-6736(00)03642-4
Low PA, Sandroni P, Joyner M, Shen WK (2009) Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol 20(3):352–358. https://doi.org/10.1111/j.1540-8167.2008.01407.x
Raj SR, Bourne KM, Stiles LE, Miglis MG, Cortez MM, Miller AJ, Freeman R, Biaggioni I, Rowe PC, Sheldon RS, Shibao CA, Diedrich A, Systrom DM, Cook GA, Doherty TA, Abdallah HI, Grubb BP, Fedorowski A, Stewart JM, Arnold AC, Pace LA, Axelsson J, Boris JR, Moak JP, Goodman BP, Chémali KR, Chung TH, Goldstein DS, Darbari A, Vernino S (2021) Postural orthostatic tachycardia syndrome (POTS): priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting—Part 2. Auton Neurosci 235:102836. https://doi.org/10.1016/j.autneu.2021.102836
Squair JW, Gautier M, Mahe L, Soriano JE, Rowald A, Bichat A, Cho N, Anderson MA, James ND, Gandar J, Incognito AV, Schiavone G, Sarafis ZK, Laskaratos A, Bartholdi K, Demesmaeker R, Komi S, Moerman C, Vaseghi B, Scott B, Rosentreter R, Kathe C, Ravier J, McCracken L, Kang X, Vachicouras N, Fallegger F, Jelescu I, Cheng Y, Li Q, Buschman R, Buse N, Denison T, Dukelow S, Charbonneau R, Rigby I, Boyd SK, Millar PJ, Moraud EM, Capogrosso M, Wagner FB, Barraud Q, Bezard E, Lacour SP, Bloch J, Courtine G, Phillips AA (2021) Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 590(7845):308–314. https://doi.org/10.1038/s41586-020-03180-w
Yavasoglu NG, Comoglu SS (2021) The effect of subthalamic deep brain stimulation on autonomic dysfunction in Parkinson’s disease: clinical and electrophysiological evaluation. Neurol Res 43(11):894–899. https://doi.org/10.1080/01616412.2021.1942409
Funding
Open Access funding enabled and organized by Projekt DEAL. Ulrich Limper received funding from the internal grant program (project IFF 2020–26) of the Faculty of Health at Witten/Herdecke University, Germany, and from the German Aerospace Center (DLR, 50WB2119) during the preparation of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jens Jordan: Serves as advisor for Novo-Nordisk, received research support from Novo-Nordisk and Boehringer Ingelheim, and is Cofounder of Eternygen GmbH.
Ulrich Limper: None.
Jens Tank: Received research support from Boston-Scientific, Novo-Nordisk, and Boehringer Ingelheim.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jordan, J., Limper, U. & Tank, J. Cardiovascular autonomic nervous system responses and orthostatic intolerance in astronauts and their relevance in daily medicine. Neurol Sci 43, 3039–3051 (2022). https://doi.org/10.1007/s10072-022-05963-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05963-7